Pharmacokinetics Dr Saed Mohammed Ibrahim 20192020 Pharmacolgy 1

Pharmacokinetics Dr. Saed Mohammed Ibrahim 2019/2020 Pharmacolgy 1
Drug metabolism • Drugs are eliminated by biotransformation and/or excretion into urine or bile • Metabolism transform lipophilic drugs into more polar products • Liver is major site of drug metabolism • Other sites kidney, intestines 2

• Some drugs are administered as inactive compound (pro-drug) & must be metabolised to their active forms • Prodrug are often designed to improve bioavailability when drug itself is poorly absorbed. • Prodrugs are used instead to improve hoe medication is absorbed, metabolized, distributed and excreted.

Prodrugs • Example : • 1 - codeine has very little activity at opioid receptor , after metabolism is converted into morphine that has greater effects at opioid receptors as analgesic. • 2 - methyldopa
Kinetics of metabolism • First-order kinetics: - Metabolism is catalyzed by enzymes - At low doses, drug metabolism is first order – rate of metabolism is directly proportional to drug dose 5 5
Kinetics of metabolism • Zero-order kinetics: - At high doses, drug metabolism is zero order- that is constant & independent of drug dose (because the enzyme is saturated by high free-drug concentration) Examples : Aspirin , ethanol , phenytoin
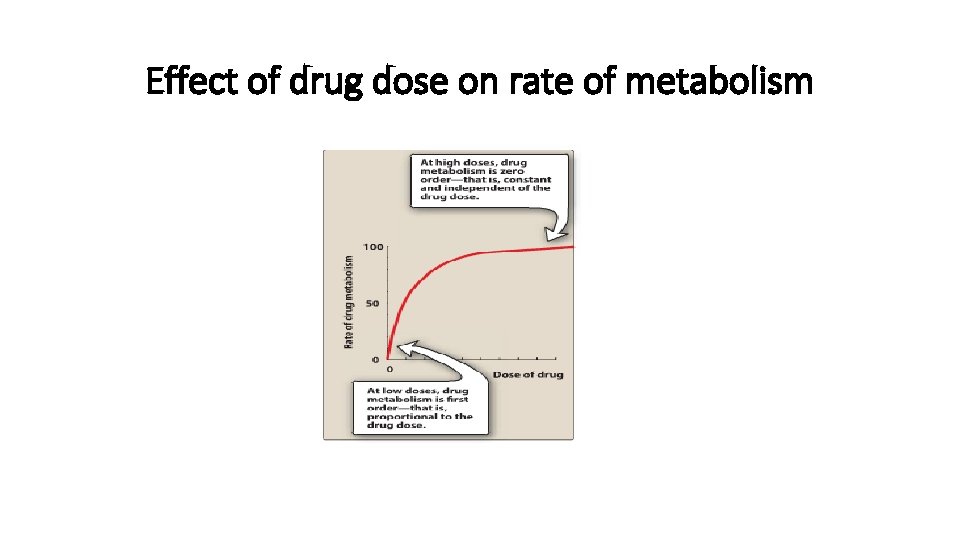
Effect of drug dose on rate of metabolism
Reactions of metabolism • Kidney cannot eliminate lipophilic drugs that readily cross cell membranes and reabsorbed in distal tubules • Lipid-soluble drugs must be metabolised in liver using two reactions called: q. Phase II 8 8
Phase I • Converts lipophilic molecules into more polar molecule by introducing or unmasking polar functional group such as –OH or –NH 2 • Phase I reactions are catalsyed by cytochrome P 450 system (also called microsomal mixed function oxidase)
Cytochrome P 450 system • Designated as CYP • Is composed of many families of heme-containg isozymes that are located in most cells primarily in liver & GI tract • There are many different genes & many different enzymes known as P 450 isoforms 10 10
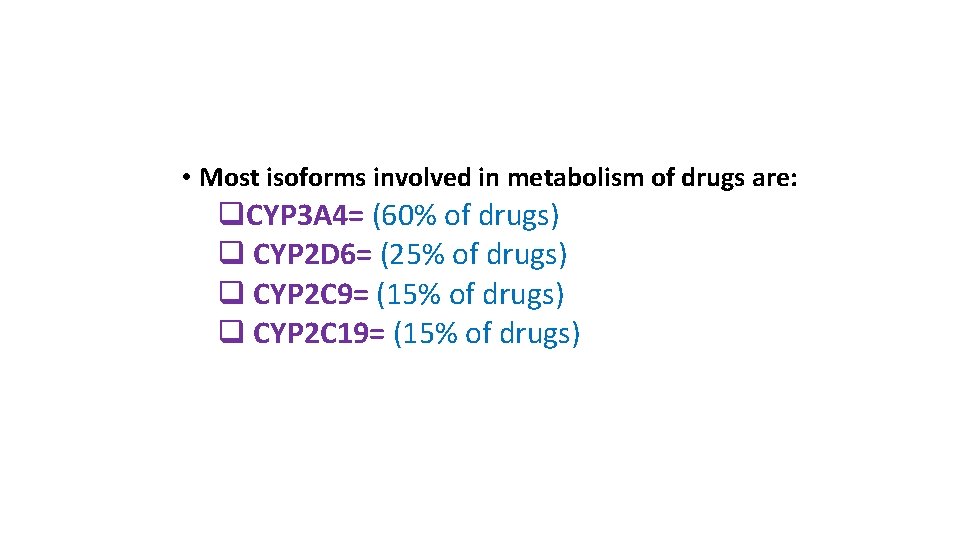
• Most isoforms involved in metabolism of drugs are: q. CYP 3 A 4= (60% of drugs) q CYP 2 D 6= (25% of drugs) q CYP 2 C 9= (15% of drugs) q CYP 2 C 19= (15% of drugs)
Genetic Variability • These enzymes exhibit genetic variability, which has implication for individual dosing regimens, determining responsiveness & risk of adverse events 12 12

• CYP 2 D 6 has been shown to exhibit genetic polymorphism • Mutations result in very low capacities to metabolize substrates • Some individuals (they lack enzyme) obtain no benefit from opioid analgesic codeine because this reaction is CYP 2 D 6 -dependent

• The frequency of this polymorphism is racially determined • A prevalence of 5 -10% in European Caucasians • Less than 2% percent of Southeast Asians
CYP 450 inducers • Inducers examples : phenobarbital, rifampin, phenytoin, carbamazepine which increase synthesis of CYP enzymes, increase metabolism of drugs by these CYP enzymes, & decrease therapeutic effect of drugs. 15 15

CYP 450 inhibitors • Inhibition of CYP isozymes can lead to serious adverse events • CYP inhibitors : 1 -erythromycin, 2 -ketoconalzole • 3 - Omeprazole is a potent inhibitor of three of CYP isozymes responsible for warfarin metabolism • 4 - cimitidine • 5 - Natural substances such as grapefruit juice may inhibit drug metabolism 16 16
Phase II • This phase consists of conjugation reactions • If the metabolite from Phase I metabolism is sufficiently polar, it can be excreted by kidneys • However, many Phase I metabolites are too lipophilic to be retained in kidney tubules

Phase II • Glucuronidation is the most common and important conjugation reaction • Drugs possessing –OH, HN 2, COOH group may enter phase II directly & become conjugated without phase I 18 18
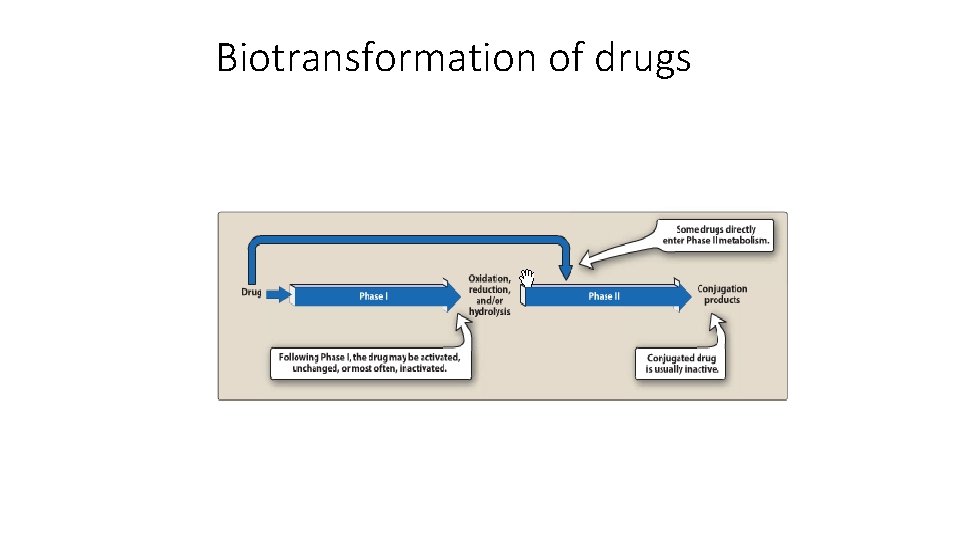
Biotransformation of drugs

Excretion or Elimination • Drug elimination: is the irreversible loss of drug from the body and occurs by two processes , metabolism, excretion. • Excretion : involves the loss of chemically unchanged drug or as polar metabolites. The main route of drug elimination is through the urine, other routes includes bile, feces, lungs, saliva, sweet, and breast milk.

Three basics processes that account for these wide differences in renal excretion 1. Glomerular filtration: most drugs, except those highly bound to plasma protein, cross the glomerular filter freely. 2. Renal tubular secretion: many drugs, especially weak acid and basic, are actively secreted into the renal tubule, and thus more rabidly excreted 21
Three basics processes that account for these wide differences in renal excretion • 3 - Passive diffusion across tubular epithelium: lipid soluble drugs are passively reabsorbed by diffusion across the tubule, so are not efficiently excreted in the urine
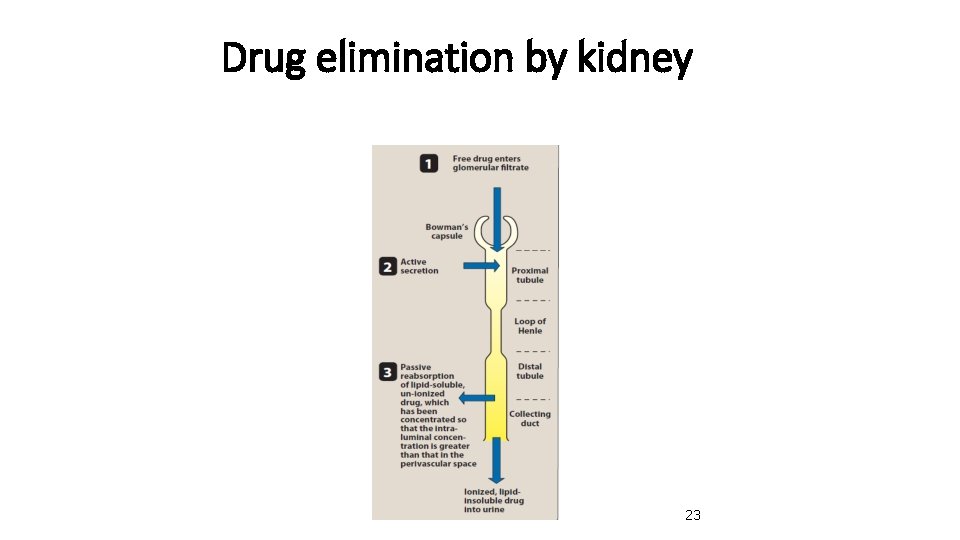
Drug elimination by kidney 23
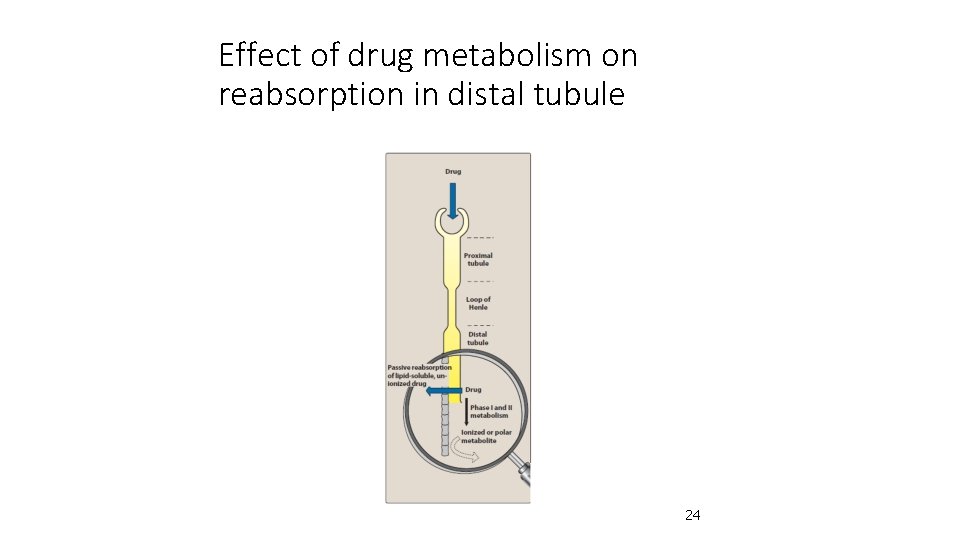
Effect of drug metabolism on reabsorption in distal tubule 24

Treatment of toxicity • As a general rule, weak acids can be eliminated by alkalinization of urine • Whereas elimination of weak bases may be increased by acidification of urine 25

• A patient presenting with phenobarbital (weak acid) overdose can be given bicarbonate, which alkalinizes urine and keeps drug ionized, • Thereby decreasing its reabsorption • If overdose is with a weak base, such as cocaine, • Acidification of urine with NH 4 Cl leads to increase in its clearance 26
Clearance • clearance is ratio of the rate elimination of a drug to its concentration in the plasma or blood • For a drug eliminated with first order kinetics, CL is a constant regardless of plasma concentration • CL depends : • 1 -the drugs • 2 -the condition of the organs of elimination in the patient • 3 - blood flow through the eliminating organ 27
- Slides: 27