Pharmacoinformatics lecture 3 Mgr Zdenk Kuera Ph D

































- Slides: 62

Pharmacoinformatics lecture 3 Mgr. Zdeněk Kučera, Ph. D. kucera. xf. cz

Evidence Based Medicine - EBM • Definition: When good quality research evidence is used in conjunction with professionals’ skills and users’/society’s perferences to solve specific problems “is the conscientious, explicit and judicious use of current best evidence in making decisions about the care of individual patients … integrating clinical expertise with the best available external evidence from systematic research” Sackett et al (1996) ------Pharmacist – self-medication, consultations with patient

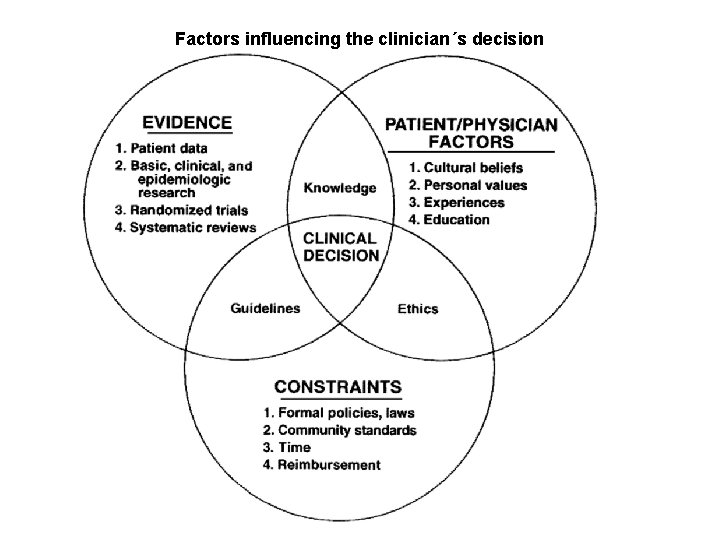
Influence factors expectation opinions finances laws evidence (EBM – not all-powerful) experience politics time

Factors influencing the clinician´s decision

Why EBM? • we are often asked for help! – we need to know, where to find relevant information • not possible to rely on more experinced colleague • not good to rely on monographs – By 1970, several studies had shown that thrombolysis (clot dissolving) drugs reduced the number of deaths from a heart attack. – The clinical value of thrombolysis. . . remains uncertain. ” Oxford Textbook of Medicine, 1987 • • postgraduate education – not systematic increase of new information, commercial information we have not time etc. . .

Advantages EBM • quick availability of new information • teach the art of critical appraisal of the literature • systematic review – new methodology of review • objective guidelines • assessment of new therapies

Criticism of the EBM • EBM is „an academic exercise“ for medical students (3 -4 questions/patient – do I have a time to search for evidence? ) • RCT – „greenhouse“ population – only because of ethics. . . ? • Governments, hospitals and HMO’s have used the jargon of EBM to justify decisions, directives, or incentives that are seen by clinicians as inappropriate. • Misuse of EBM by pharmaceutical companies – „half-truths“ • treatment of the patient (co-morbidity, polypragmasia, aging, lactation, gravidity, pediatrics) • EBM is seen by some to promote “therapeutically nihilistic” approaches to medical practice since critical appraisal of commonly used therapies often concludes that they provide little or no benefit – e. g. cough - Over the counter cough medicines for acute cough cannot be recommended because there is no good evidence for their effectiveness. BMJ 2002; 324: 329 • Guidelines – impersonal, schematic. . . • No evidence – no treatment? ? ? • If I do not treat according to guidelines – see you in a court?

Evidence-based practice 5 STEPS: • Formulate a good question • Look for evidence • Critically appraise what you find • Act on the evidence • Evaluate the results

Step one: Formulating questions

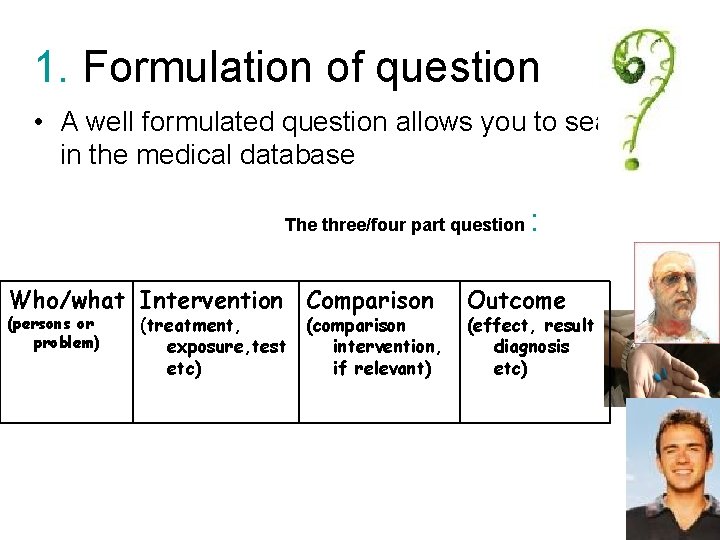
1. Formulation of question • A well formulated question allows you to search in the medical database The three/four part question Who/what Intervention Comparison (persons or problem) (treatment, exposure, test etc) (comparison intervention, if relevant) : Outcome (effect, result diagnosis etc)

What is a good question?

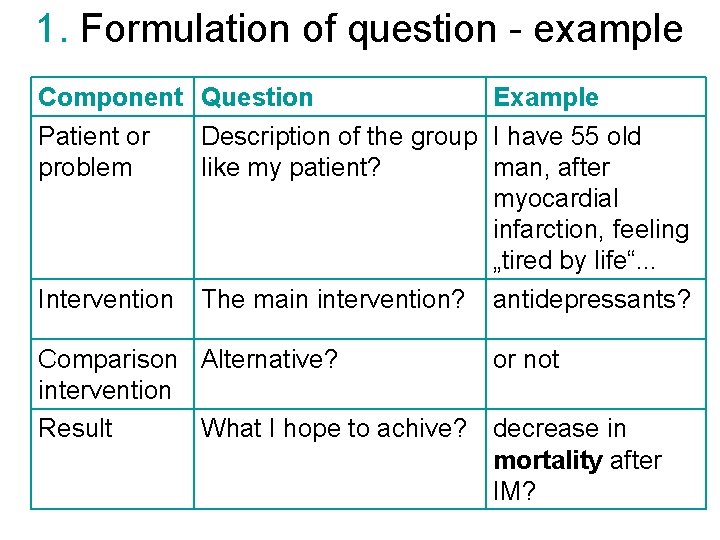
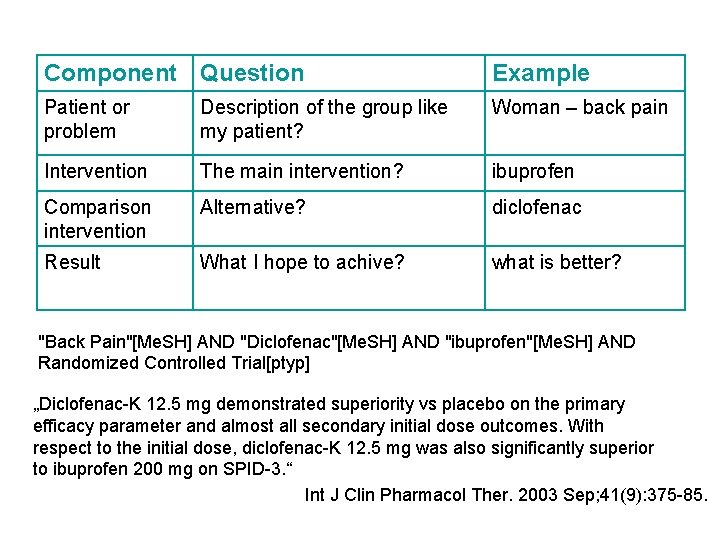
1. Formulation of question - example Component Question Example Patient or Description of the group I have 55 old problem like my patient? man, after myocardial infarction, feeling „tired by life“. . . Intervention The main intervention? antidepressants? Comparison Alternative? or not intervention Result What I hope to achive? decrease in mortality after IM?

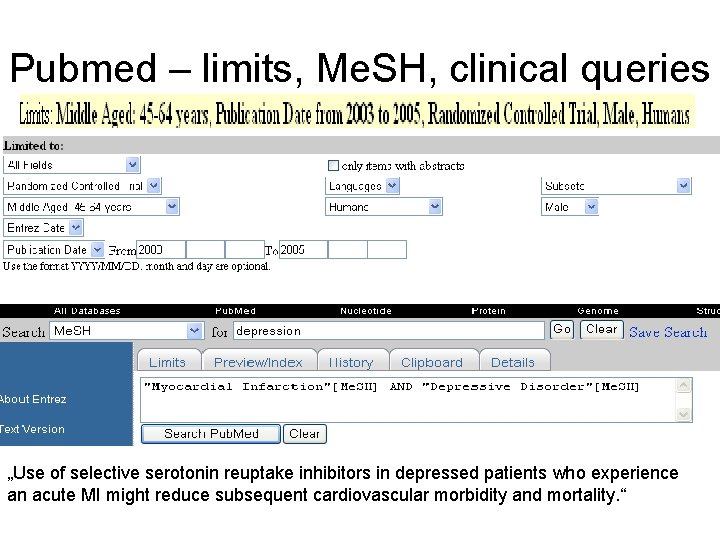
Pubmed – limits, Me. SH, clinical queries „Use of selective serotonin reuptake inhibitors in depressed patients who experience an acute MI might reduce subsequent cardiovascular morbidity and mortality. “

Component Question Example Patient or problem Description of the group like my patient? Woman – back pain Intervention The main intervention? ibuprofen Comparison intervention Alternative? diclofenac Result What I hope to achive? what is better? "Back Pain"[Me. SH] AND "Diclofenac"[Me. SH] AND "ibuprofen"[Me. SH] AND Randomized Controlled Trial[ptyp] „Diclofenac-K 12. 5 mg demonstrated superiority vs placebo on the primary efficacy parameter and almost all secondary initial dose outcomes. With respect to the initial dose, diclofenac-K 12. 5 mg was also significantly superior to ibuprofen 200 mg on SPID-3. “ Int J Clin Pharmacol Ther. 2003 Sep; 41(9): 375 -85.

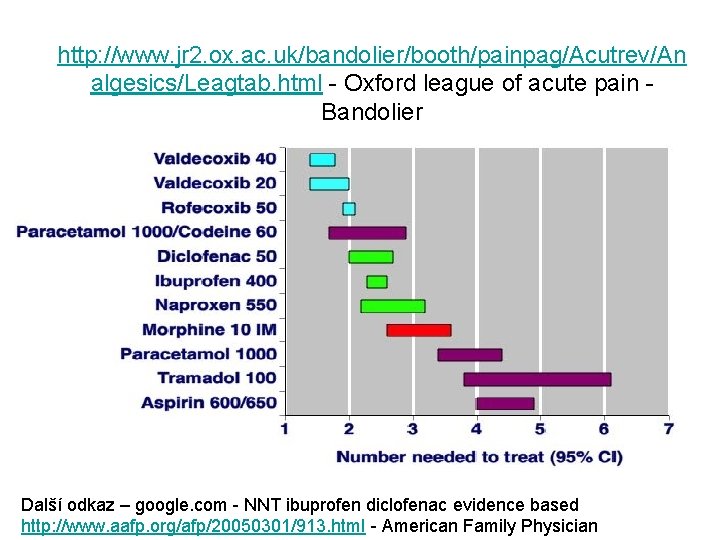
http: //www. jr 2. ox. ac. uk/bandolier/booth/painpag/Acutrev/An algesics/Leagtab. html - Oxford league of acute pain - Bandolier Další odkaz – google. com - NNT ibuprofen diclofenac evidence based http: //www. aafp. org/afp/20050301/913. html - American Family Physician

What kind of question did you formulate? • How many people have a this problem …. ? • Why do some people get this problem. . ? • How can we decide whether someone has this problem…? • What can we do to prevent or cure. . ? • What is the prognosis ……? • How does it feel to …. ? • How can we understand …. . ?

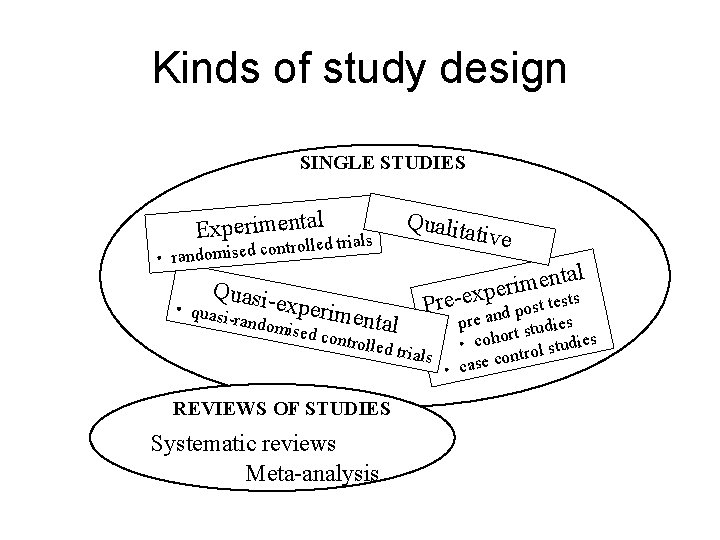
Kinds of study design SINGLE STUDIES Experimentald trials controlle d e is m o d n a r • Quasi- Qualita ti ve l a t n e m ri e p x e sts Pre post te exp • quasi -random erimental e and udies r p • ised co rt st o h ntrolled o c • tudies s l trials o r t con • case REVIEWS OF STUDIES Systematic reviews Meta-analysis

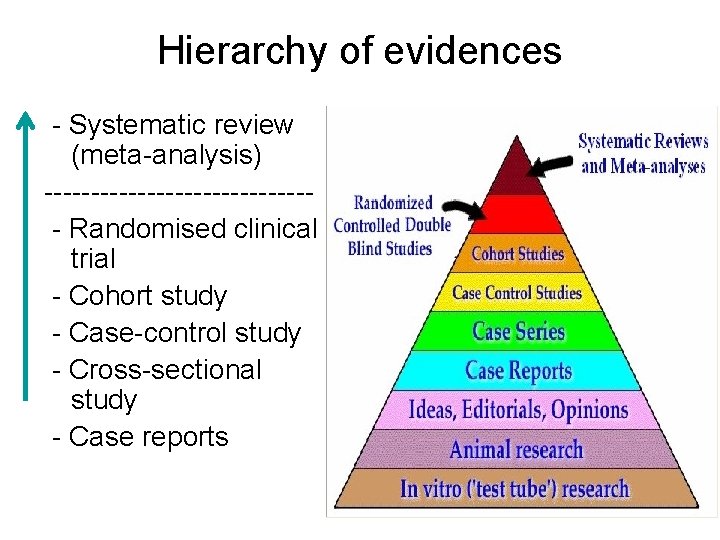
Hierarchy of evidences - Systematic review (meta-analysis) -------------- - Randomised clinical trial - Cohort study - Case-control study - Cross-sectional study - Case reports

Case reports • Description of disease, treatment, phenomenon in one selected patient • Can be summarised into case-series Czech Republic: http: //www. zdn. cz/kazuistiky

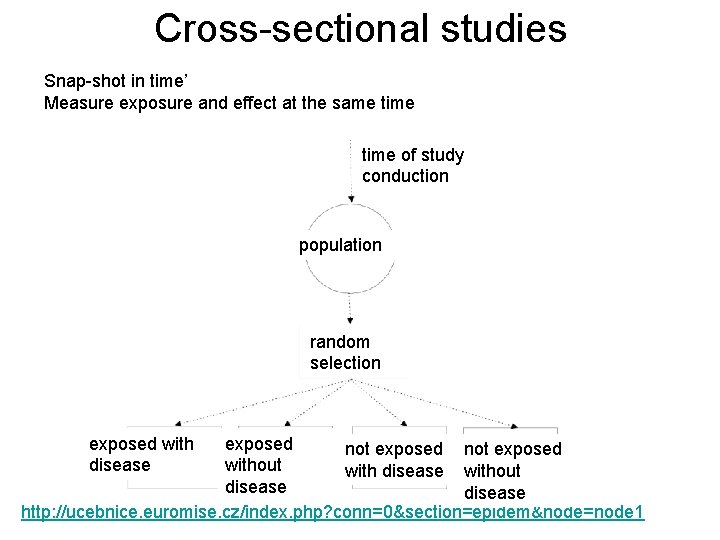
Cross-sectional studies Snap-shot in time’ Measure exposure and effect at the same time of study conduction population random selection exposed with disease exposed not exposed without with disease without disease http: //ucebnice. euromise. cz/index. php? conn=0§ion=epidem&node=node 1

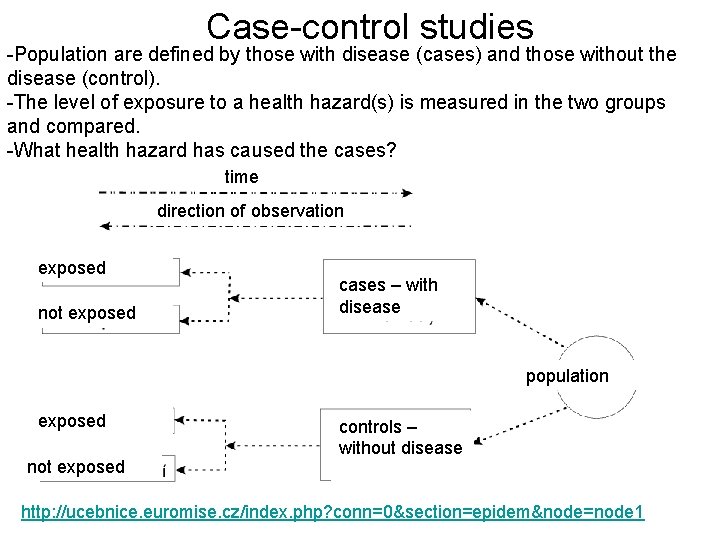
Case-control studies -Population are defined by those with disease (cases) and those without the disease (control). -The level of exposure to a health hazard(s) is measured in the two groups and compared. -What health hazard has caused the cases? time direction of observation exposed not exposed cases – with disease population exposed not exposed controls – without disease http: //ucebnice. euromise. cz/index. php? conn=0§ion=epidem&node=node 1

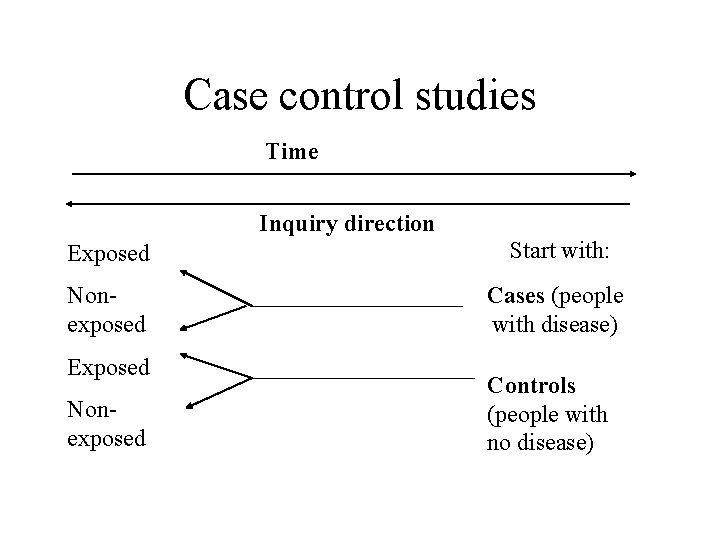
Case control studies Time Inquiry direction Exposed Start with: Nonexposed Cases (people with disease) Exposed Nonexposed Controls (people with no disease)

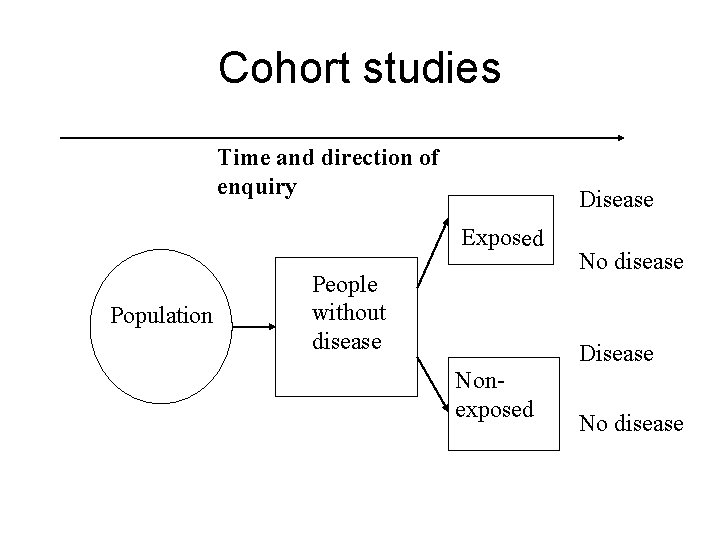
Cohort studies • The population of study is defined by exposure to a health hazard • They are followed-up over time to observe incidence of disease in exposed and nonexposed

Cohort studies Time and direction of enquiry Disease Exposed Population People without disease No disease Disease Nonexposed No disease

A cohort study A cohort (or follow-up or longitudinal) study is when a group of people exposed and a group of persons unexposed to a potential cause of disease are followed-up over time and the incidence of the disease in one group compared with the incidence in the other.

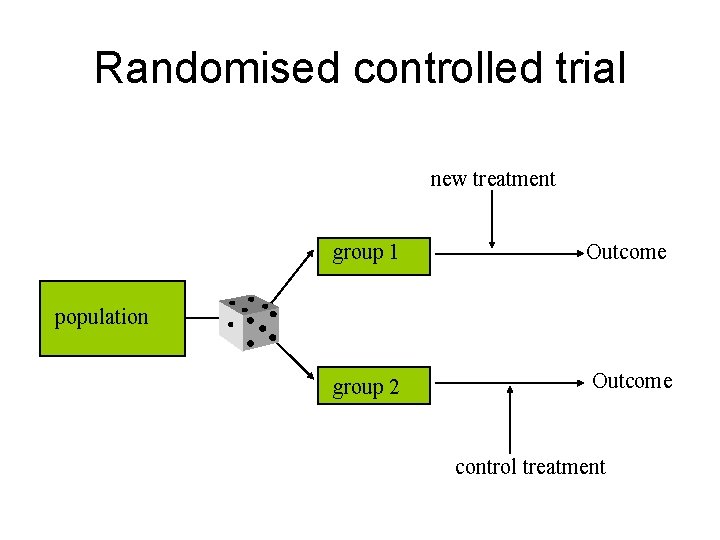
Randomised controlled trial new treatment group 1 Outcome group 2 Outcome population control treatment

RCT – randomized clinical trial Clinical - experimental study on patient Controlled – experimental drug or procedure are compared with another drug, procedure, or placebo Randomized - patients are divided into groups randomly Double blind – patient and doctor does not know what drug is used Intention-to-treat – calculating the results with the number of patients in the beginning of the study Check: • Diagnostic or screening tests • Treatment technique • Effectiveness and safety of of drug treatment

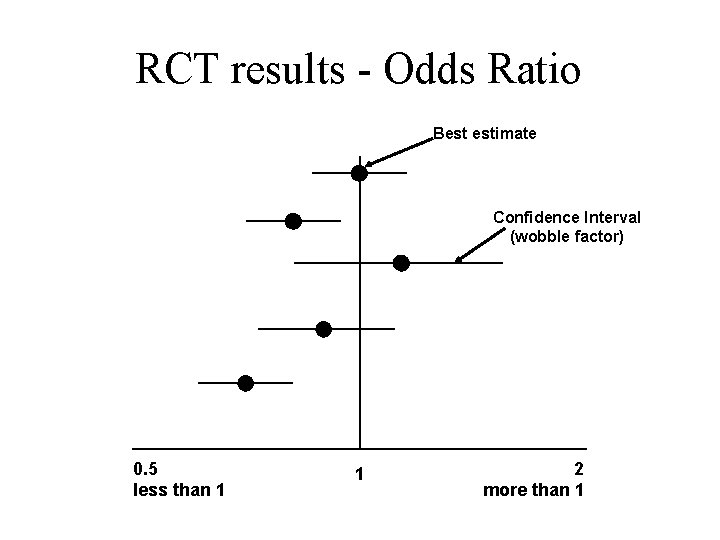
RCT results - Odds Ratio Best estimate Confidence Interval (wobble factor) 0. 5 less than 1 1 2 more than 1

Numbers needed to treat • Number of people on average needed to receive treatment to produce one additional beneficial outcome • http: //www. healthcare. ubc. ca/calc/clinsig. h tml

Confidence Interval (CI) = the ‘uncertainty’ factor, how sure are we about the results? - the shorter the CI the more certain we are about the results - if it crosses the line of 1 (no treatment effect) the intervention might not be doing any good and could be doing harm

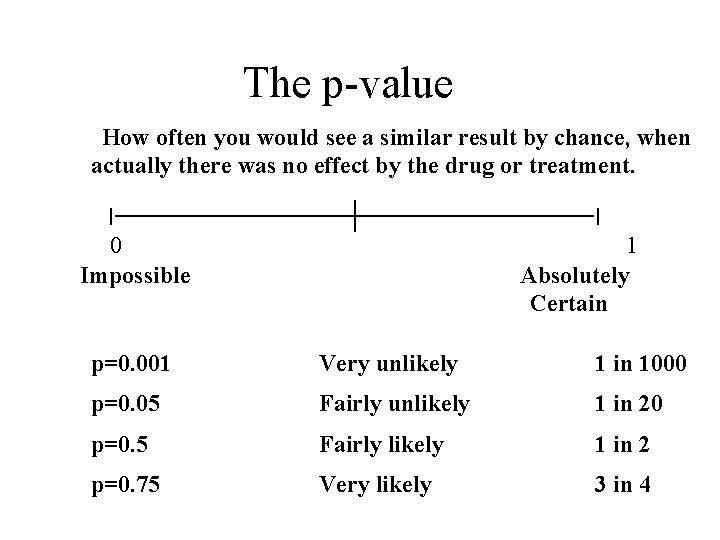
The p-value How often you would see a similar result by chance, when actually there was no effect by the drug or treatment. 0 Impossible 1 Absolutely Certain p=0. 001 Very unlikely 1 in 1000 p=0. 05 Fairly unlikely 1 in 20 p=0. 5 Fairly likely 1 in 2 p=0. 75 Very likely 3 in 4

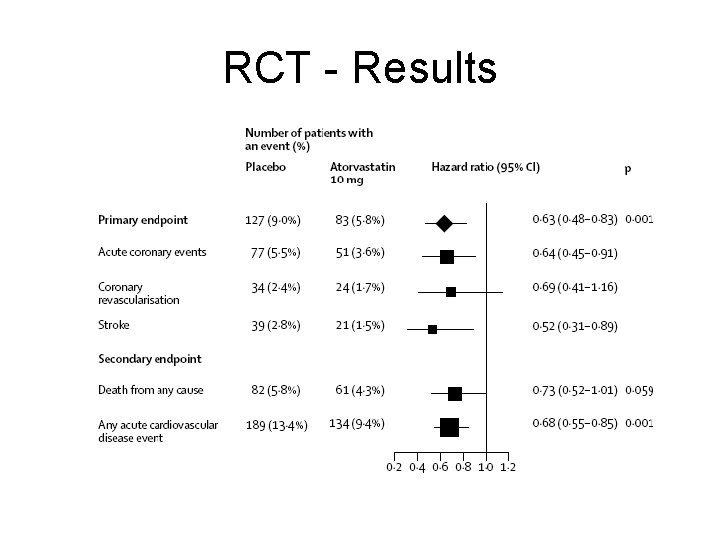
RCT - Results

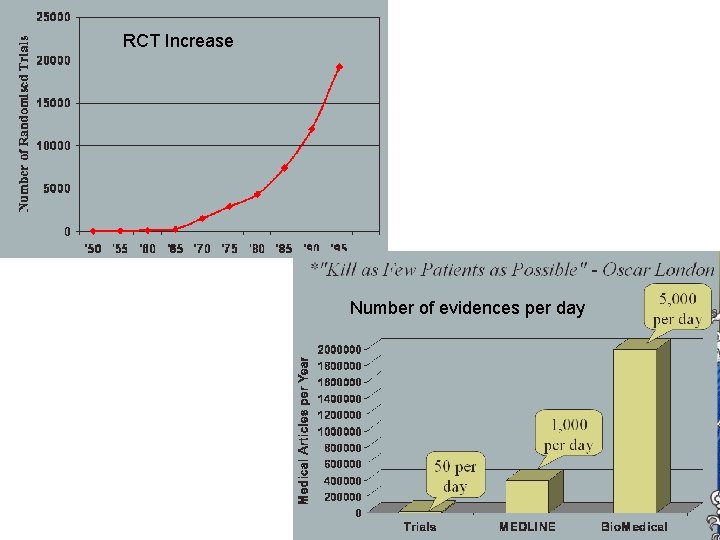
RCT Increase Number of evidences per day

Why bother with reviews? • volume of literature is condensed • “new” information is made accessible • some reviews are extremely good and take considerable time to find all the information on one topic

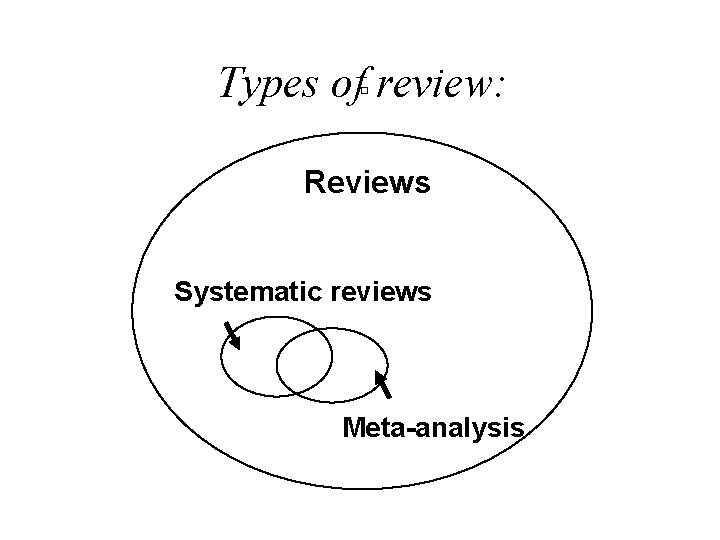
Types of review: � Reviews Systematic reviews Meta-analysis

“The medical literature can be compared to a jungle. It is fast growing, full of dead wood, sprinkled with hidden treasure and infested with spiders and snakes. ” Peter Morgan, Scientific Editor, Canadian Medical Association

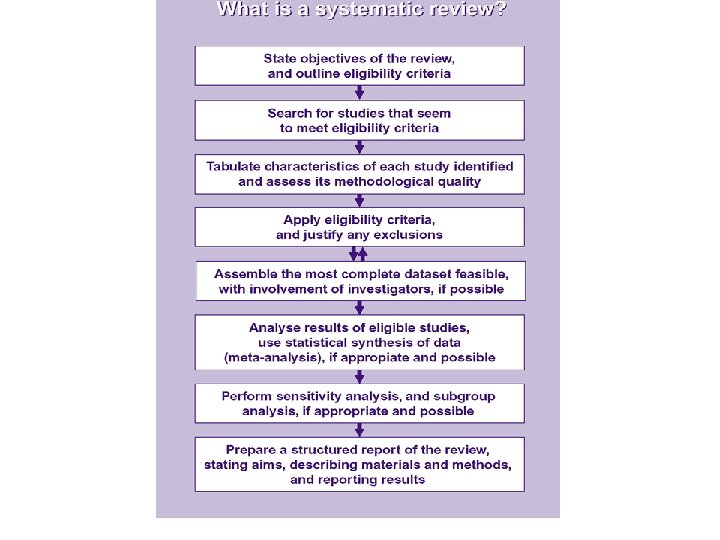
Systematic Review • Review of all literature • On particular topic • Systematically identified, appraised and summarised


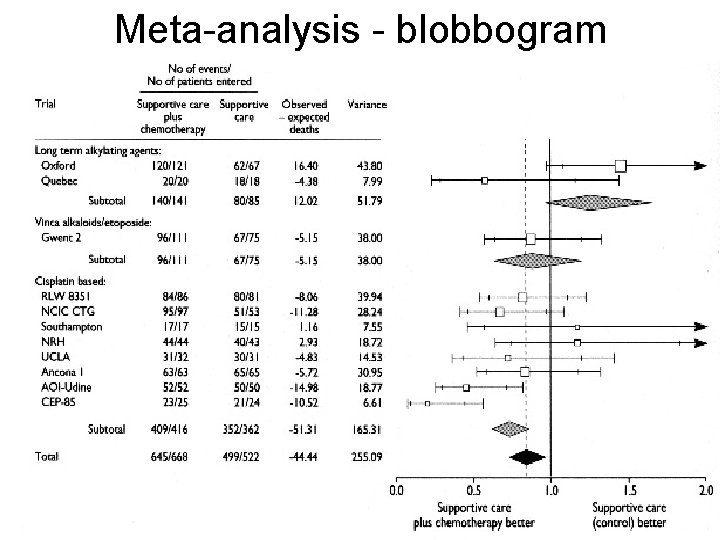
Meta-analysis • Statistical synthesis of results of several studies, which dealt with the same question

Odds Ratio Graph (Blobbogram) LEFT E S S M O RIGHT E Line of no significance 0. 5 less than 1 1 2 more than 1

Odds Ratio (Blobbogram) 0. 5 less than 1 1 2 more than 1

Meta-analysis - blobbogram

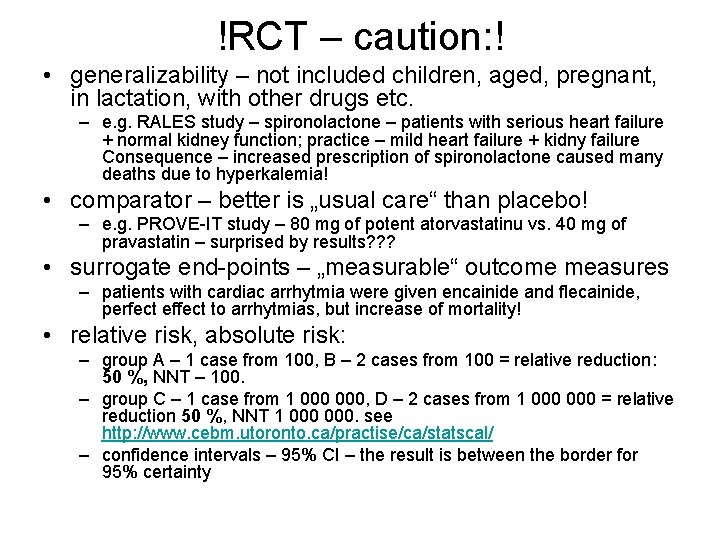
!RCT – caution: ! • generalizability – not included children, aged, pregnant, in lactation, with other drugs etc. – e. g. RALES study – spironolactone – patients with serious heart failure + normal kidney function; practice – mild heart failure + kidny failure Consequence – increased prescription of spironolactone caused many deaths due to hyperkalemia! • comparator – better is „usual care“ than placebo! – e. g. PROVE-IT study – 80 mg of potent atorvastatinu vs. 40 mg of pravastatin – surprised by results? ? ? • surrogate end-points – „measurable“ outcome measures – patients with cardiac arrhytmia were given encainide and flecainide, perfect effect to arrhytmias, but increase of mortality! • relative risk, absolute risk: – group A – 1 case from 100, B – 2 cases from 100 = relative reduction: 50 %, NNT – 100. – group C – 1 case from 1 000, D – 2 cases from 1 000 = relative reduction 50 %, NNT 1 000. see http: //www. cebm. utoronto. ca/practise/ca/statscal/ – confidence intervals – 95% CI – the result is between the border for 95% certainty

!RCT – caution: ! • statistical vs clinical relevance – statistical significance does not neccessarily mean clinical significance (more patients – better statistical significance) • Side effects „overlook“ – COX 2 cardiotoxic, but Merck (rofecoxib) does not care for years, COX 2 prevented 5 ulcers from 1000 patients, but caused 6 cardiovascular events Side effects with frequency 1: 1000 statistically undetectable. • combined measures in „primary“ outcome of study – UKPDS – 1 „event“ = 21 different parameters (death, infarction, nefropathy. . . ) • primary end-point not significant, secondary significant – p = 0, 05 – 1 from 20 repetition can be mistake and I admit this! If 20 results – 1 mistake very presumable • class effect – studie HOPE, PEACE, EUROPA – ACEI are not the same! • influence of pharmaceutical companies – They fund so they often adjudicate the publication or analyse the data! Negative result publication bias. . . NEJM 352: 2202 -2210 – 50 % of CRO accept companies advices for publication

Step two: Finding Evidence

2. Finding the Evidence EBM books EBM journals EBM databases EBM Guidelines Now merged with ACP

ACP Journal Club – see bi. cuni. cz ABC Index EBSCO or Evidence Based Medicine Reviews

Cochrane collaboration • International non-profit independent organisation – main aim – development of Cochrane Library • Archie Cochrane – founder • Why? Too much information, is there the possibility to sort? Aim – development of the database of systematic review • 90 countries – different centres – language support • Collaborative Review Groups – development and maintenance of systematic reviews • Mainly volunteers • http: //www. cochrane. org/index 0. htm

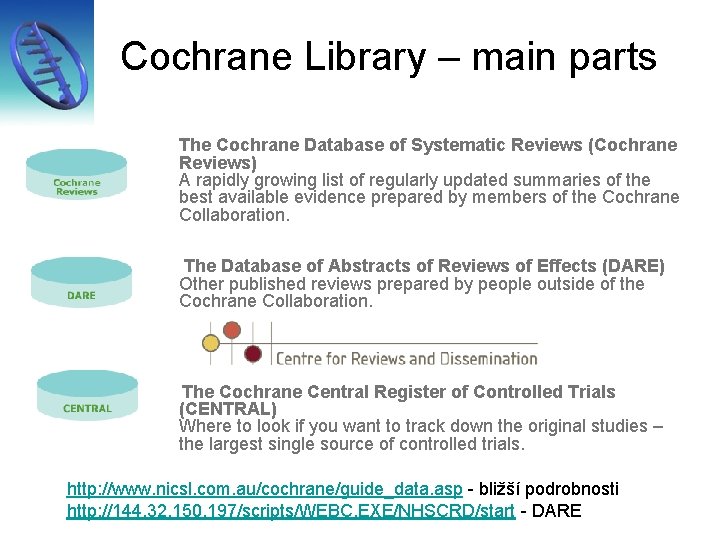
Cochrane Library – main parts The Cochrane Database of Systematic Reviews (Cochrane Reviews) A rapidly growing list of regularly updated summaries of the best available evidence prepared by members of the Cochrane Collaboration. The Database of Abstracts of Reviews of Effects (DARE) Other published reviews prepared by people outside of the Cochrane Collaboration. The Cochrane Central Register of Controlled Trials (CENTRAL) Where to look if you want to track down the original studies – the largest single source of controlled trials. http: //www. nicsl. com. au/cochrane/guide_data. asp - bližší podrobnosti http: //144. 32. 150. 197/scripts/WEBC. EXE/NHSCRD/start - DARE

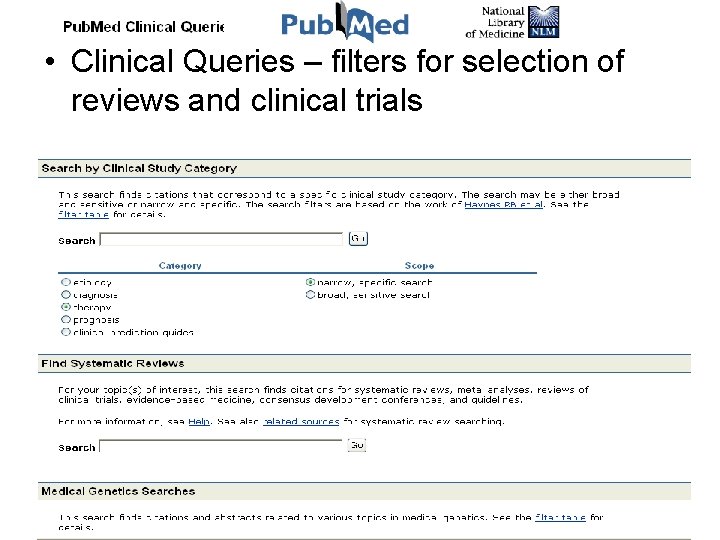
• Clinical Queries – filters for selection of reviews and clinical trials

TRIP database (Turning Research into Practice) • http: //www. tripdatabase. com/ • TRIP searches a broad range of evidencebased health care-related websites including Bandolier (good for allied health) and Cochrane Library databases. Simple search. View articles one by one

SUMSearch “that was some search!” • http: //sumsearch. uthscsa. edu/ • Devised by the University of Texas Health Sciences Center at San Antonio. • Searches MEDLINE, DARE, an on-line textbook, usually the Merck manual, etc. • Uses Me. SH thesaurus

guidelines • Document „How to treat“ based on the newest evidences • Czech Republic: – http: //www. cls. cz/dp/index. htm CLS JEP – http: //www. svl. cz/default. aspx/cz/spol/svl/default/men u/doporucenepostu SVL ----- • World: – National Guideline Clearinghouse - http: //www. guideline. gov/ - USA – NICE - http: //www. nice. org. uk/ - UK – NHS – GIN – www. g-i-n. net – International network – The Agency for Quality in Medicine (AQUMED) – www. leitlinien. de – BRD – http: //www. phac-aspc. gc. ca/dpg_e. html - Canada – etc. . . !

Step three: Critical appraisal

3. Critical appraisal • Case report < case-control study < cohort study < RCT < systematic review (< guideline) • CASP – critical appraisal skills programme http: //www. phru. nhs. uk/casp/critical_appra isal_tools. htm

Step four: Implementing evidence

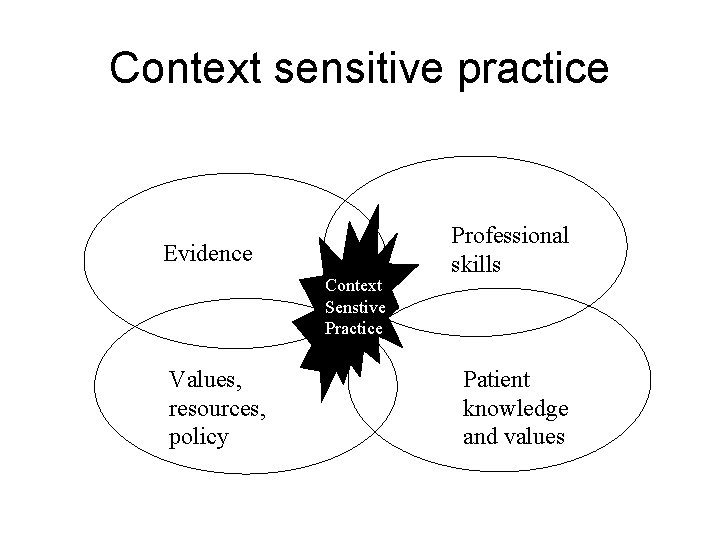
Context sensitive practice Evidence Context Senstive Sensitive Practice Values, resources, policy Professional skills Patient knowledge and values

Step five: Evaluating results

Evaluation Practice can be evaluate through • your own reflection • quality assurance programmes • further research

Drug Information Centres (DIC) • http: //www. napra. org/docs/0/95/157/164. a sp - Canada - list • http: //www. uh. edu/~libn/drug. htm - example of information sources in DIC • http: //www. shpa. org. au/docs/druginfo_int. h tml - !international register! of Drug Information Centres

DIC CR – see Solutio – www. medon. cz • Informace o farmaceutických přípravcích: Farmaceutický informační servis (Phoenix) Vinohradská 72, 618 00 Brno tel. 548 135 171 v. fricova@bm. phoenix. cz • Lékové informační centrum (LIC) Lékárna FN U sv. Anny v Brně Pekařská 53, 656 91 Brno tel. 543 182 175 -7 tel. /fax 543 211 429 premysl. cerny@fnusa. cz pavla. kuksova@fnusa. cz • Lékové informační centrum Katedra sociální a klinické farmacie, Fa. F UK v Hradci Králové Heyrovského 1203, 500 05 Hradec Králové 5 tel. 495 067 452 fax 495 512 266 lic@faf. cuni. cz • Středisko vědeckých informací (SUKL) Šrobárova 48, 100 41 Praha 10 tel. 272 185 829, 272 185 333 fax 272 185 756 svi@sukl. cz, infs@sukl. cz • Lékové informační centrum při 3. LF MUDr. Martin Votava Ústav farmakologie 3. LF UK Ruská 87 100 00, Praha 10 www. zdravcentra. cz – votava@farmakologie. net formulář pro vložení dotazu

Drug agencies… • Czech Republic – www. sukl. cz • EU – www. emea. eu. int • see „human medicines“ • requires JAVAscript! • EPAR – better than SPC! • USA – www. fda. gov • CDER -----USA – see http: //www. cdc. gov/ ! – description of different diseases