Pharmacogenetics Pharmcogenomics Russ B Altman MD Ph D
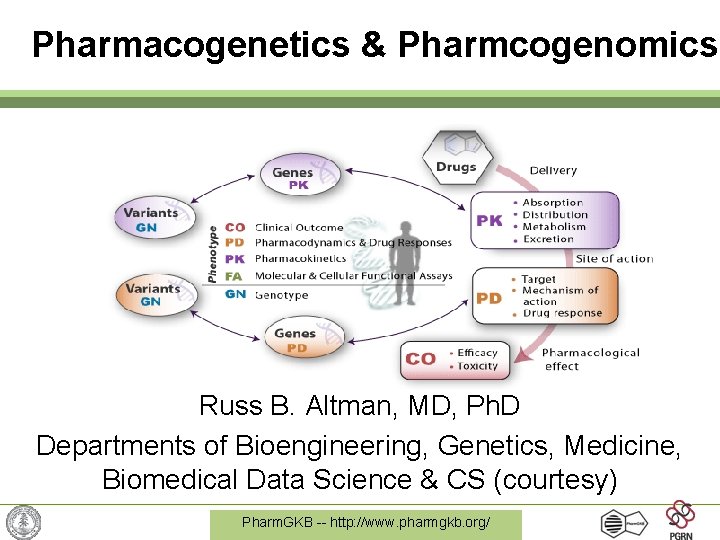
Pharmacogenetics & Pharmcogenomics Russ B. Altman, MD, Ph. D Departments of Bioengineering, Genetics, Medicine, Biomedical Data Science & CS (courtesy) Pharm. GKB -- http: //www. pharmgkb. org/

Pharmacogenetics is Defined “The role of genetics in drug responses. ” F. Vogel, 1959 Pharm. GKB -- http: //www. pharmgkb. org/ CP 1077369 -1

January 15, 2001 Pharm. GKB -- http: //www. pharmgkb. org/
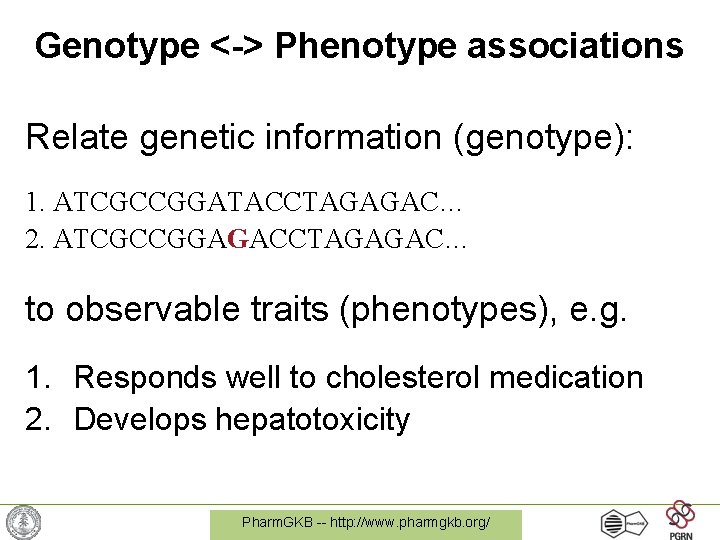
Genotype <-> Phenotype associations Relate genetic information (genotype): 1. ATCGCCGGATACCTAGAGAC… 2. ATCGCCGGAGACCTAGAGAC… to observable traits (phenotypes), e. g. 1. Responds well to cholesterol medication 2. Develops hepatotoxicity Pharm. GKB -- http: //www. pharmgkb. org/
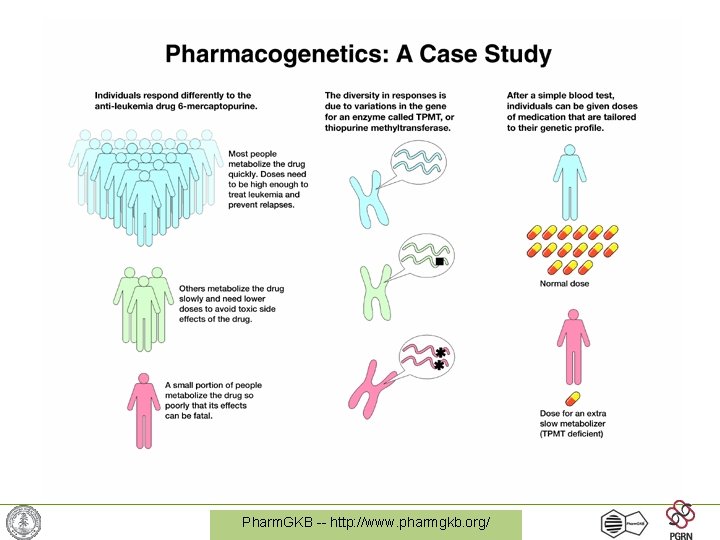
Pharm. GKB -- http: //www. pharmgkb. org/
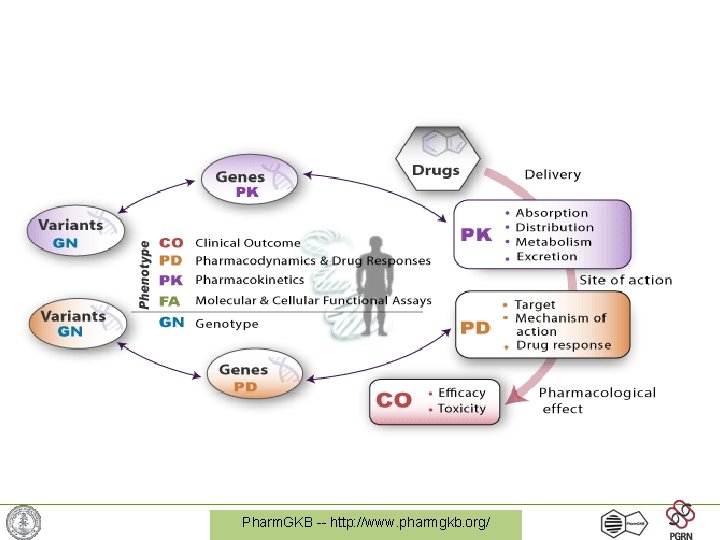
Pharm. GKB -- http: //www. pharmgkb. org/
Purine analogs • 6 -mercaptopurine, 6 -thioguanine, azathioprine • Used to treat lymphoblastic leukemia, autoimmune disease, inflammatory bowel disease, after transplant • Interferes with nucleic acid synthesis • Therapeutic index limited by myelosuppression Pharm. GKB -- http: //www. pharmgkb. org/
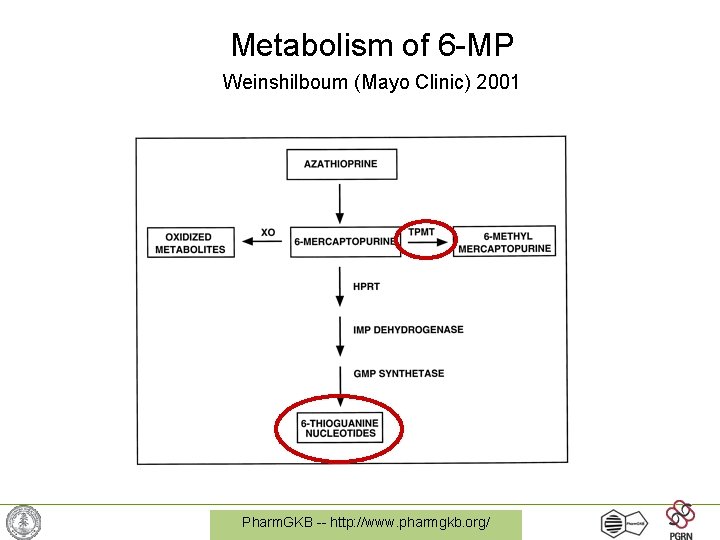
Metabolism of 6 -MP Weinshilboum (Mayo Clinic) 2001 Pharm. GKB -- http: //www. pharmgkb. org/
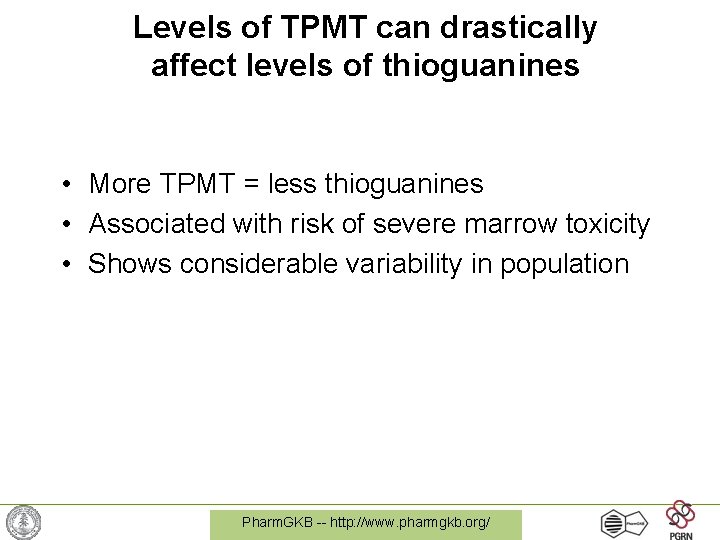
Levels of TPMT can drastically affect levels of thioguanines • More TPMT = less thioguanines • Associated with risk of severe marrow toxicity • Shows considerable variability in population Pharm. GKB -- http: //www. pharmgkb. org/
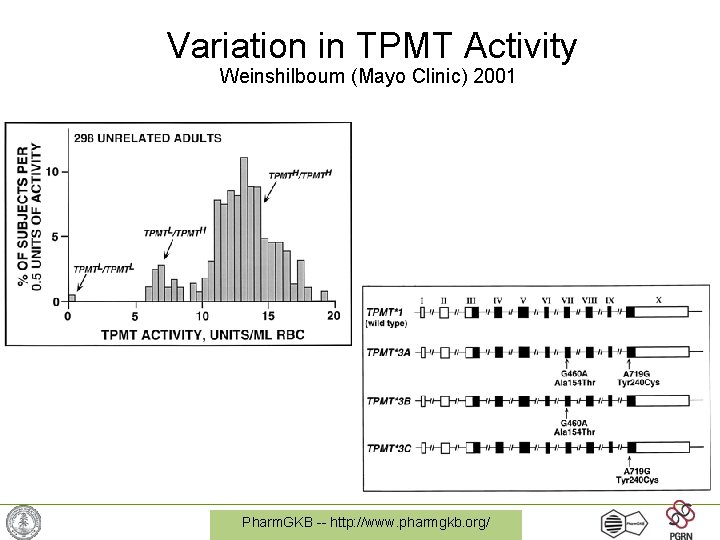
Variation in TPMT Activity Weinshilboum (Mayo Clinic) 2001 Pharm. GKB -- http: //www. pharmgkb. org/
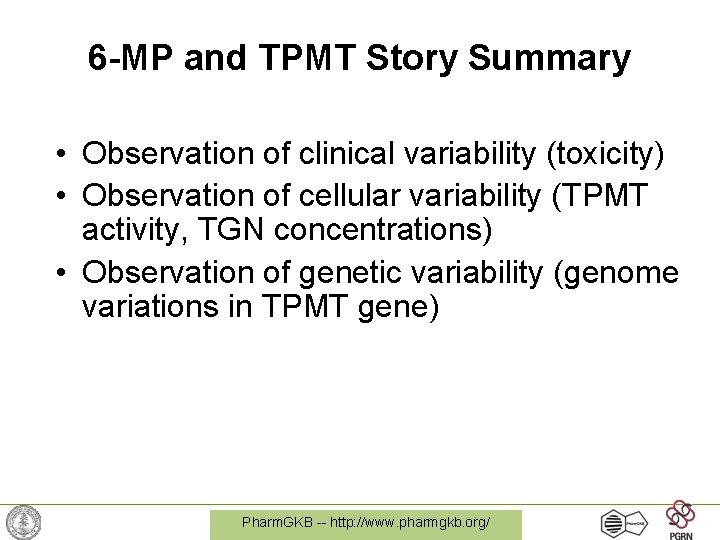
6 -MP and TPMT Story Summary • Observation of clinical variability (toxicity) • Observation of cellular variability (TPMT activity, TGN concentrations) • Observation of genetic variability (genome variations in TPMT gene) Pharm. GKB -- http: //www. pharmgkb. org/
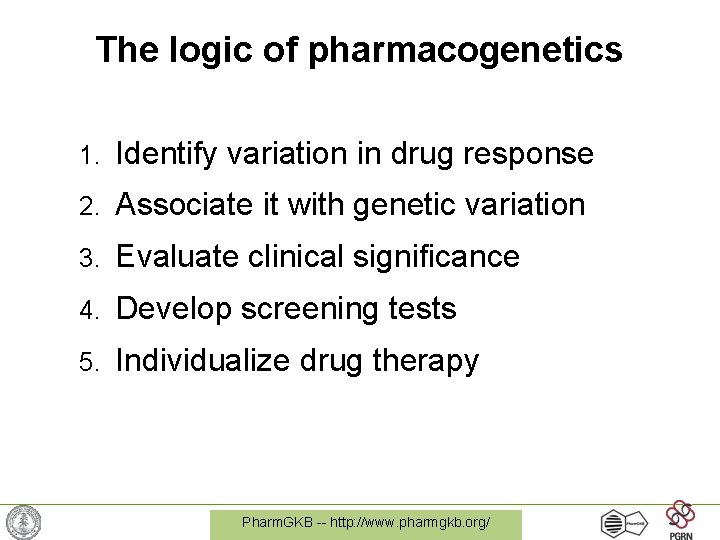
The logic of pharmacogenetics 1. Identify variation in drug response 2. Associate it with genetic variation 3. Evaluate clinical significance 4. Develop screening tests 5. Individualize drug therapy Pharm. GKB -- http: //www. pharmgkb. org/

What is the clinical promise? • Focused treatment by pre-identifying genetic backgrounds likely to respond • Reduce adverse events by predicting who is at risk • A way to save drugs in the pipeline that are very effective only in subpopulations • Better understanding of drug interactions Pharm. GKB -- http: //www. pharmgkb. org/
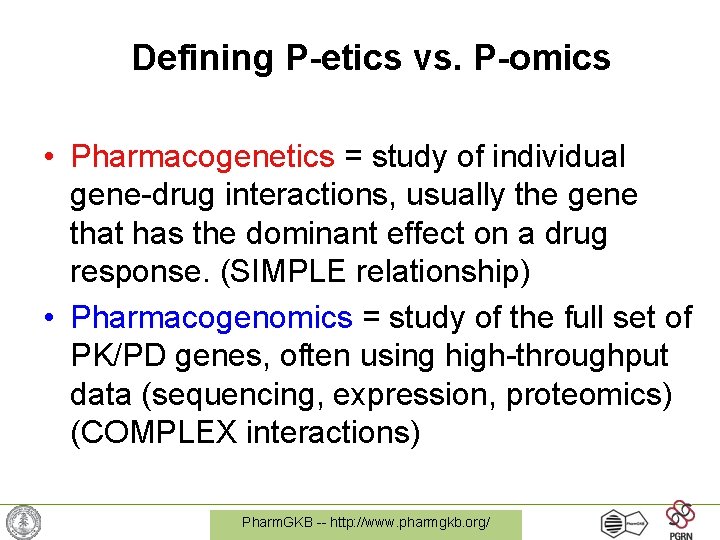
Defining P-etics vs. P-omics • Pharmacogenetics = study of individual gene-drug interactions, usually the gene that has the dominant effect on a drug response. (SIMPLE relationship) • Pharmacogenomics = study of the full set of PK/PD genes, often using high-throughput data (sequencing, expression, proteomics) (COMPLEX interactions) Pharm. GKB -- http: //www. pharmgkb. org/
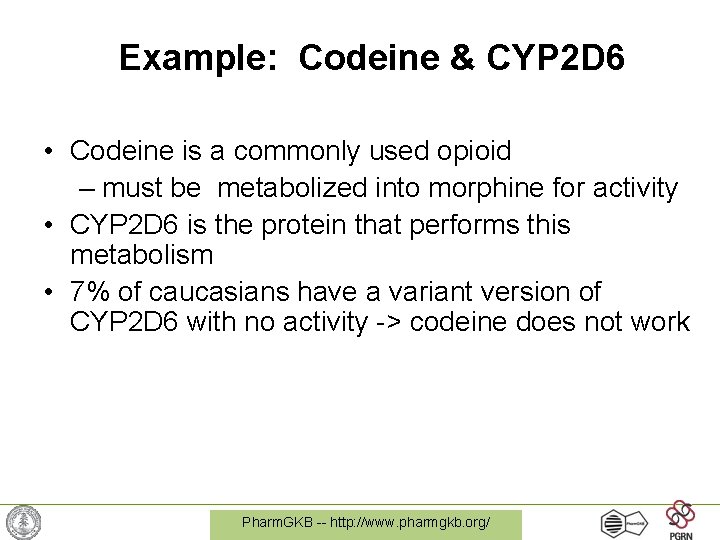
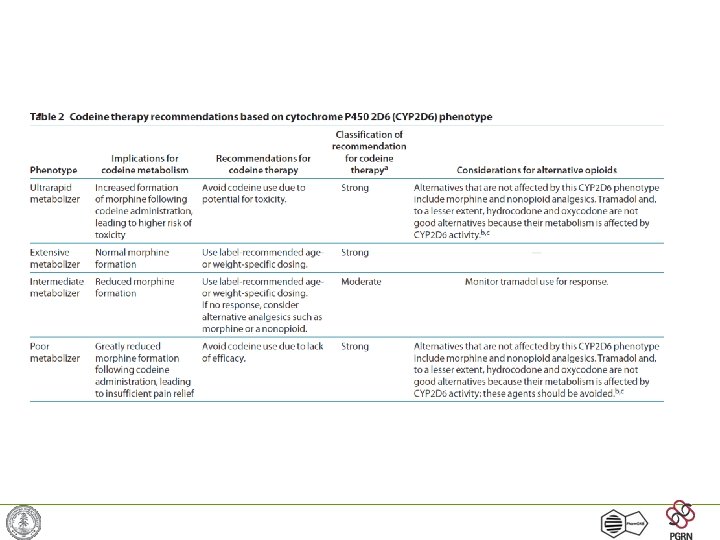
Example: Codeine & CYP 2 D 6 • Codeine is a commonly used opioid – must be metabolized into morphine for activity • CYP 2 D 6 is the protein that performs this metabolism • 7% of caucasians have a variant version of CYP 2 D 6 with no activity -> codeine does not work Pharm. GKB -- http: //www. pharmgkb. org/
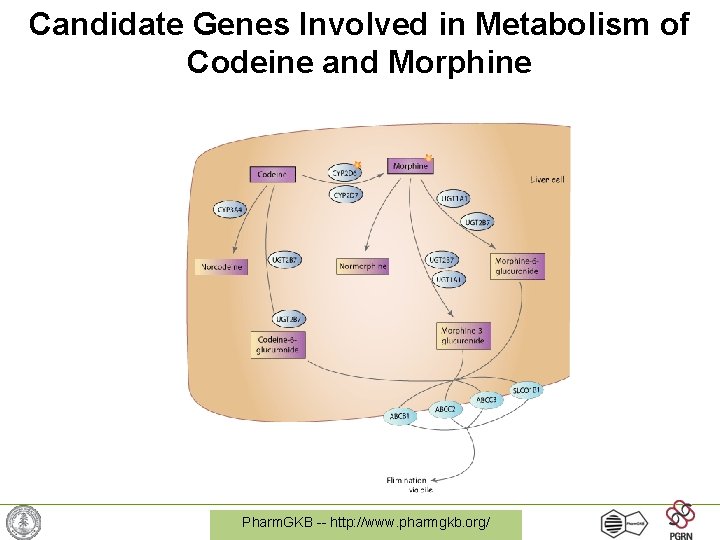
Candidate Genes Involved in Metabolism of Codeine and Morphine Pharm. GKB -- http: //www. pharmgkb. org/
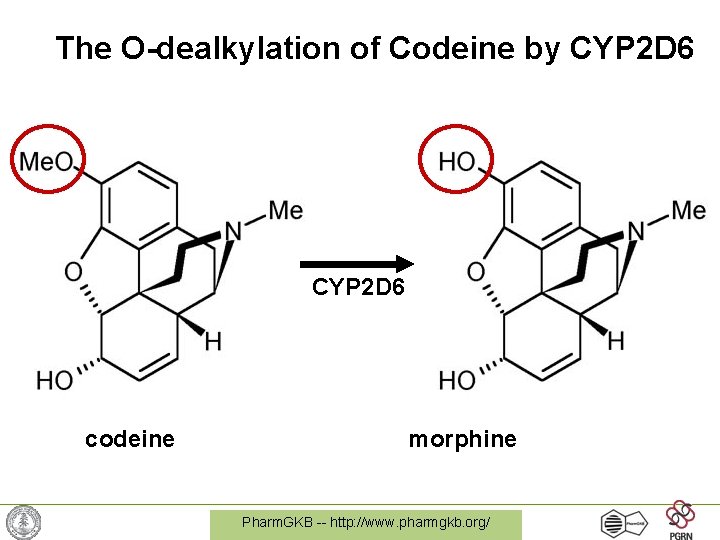
The O-dealkylation of Codeine by CYP 2 D 6 codeine morphine Pharm. GKB -- http: //www. pharmgkb. org/
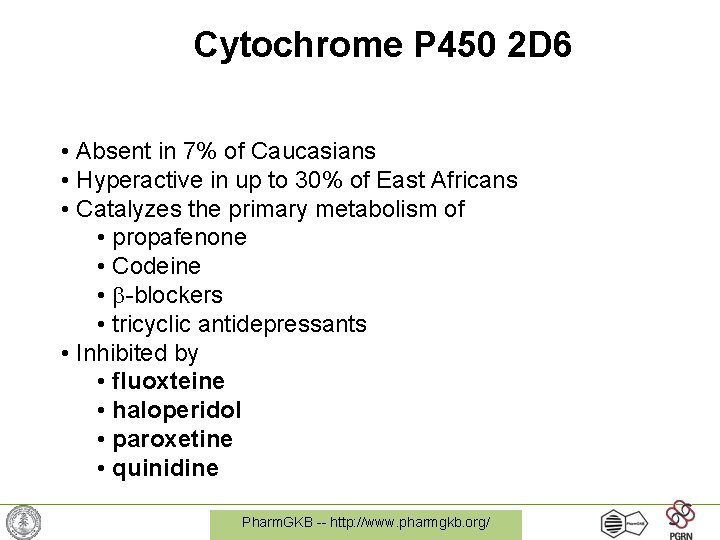
Cytochrome P 450 2 D 6 • Absent in 7% of Caucasians • Hyperactive in up to 30% of East Africans • Catalyzes the primary metabolism of • propafenone • Codeine • -blockers • tricyclic antidepressants • Inhibited by • fluoxteine • haloperidol • paroxetine • quinidine Pharm. GKB -- http: //www. pharmgkb. org/

CYP 2 D 6 Alleles • 125 alleles as of October 2008 • 10 alleles where haplotype has not been determined • 38 alleles have no activity • 10 alleles have decreased activity • The *2 variant can have 1, 2, 3, 4, 5 or 13 copies resulting in increased activity http: //www. cypalleles. ki. se/cyp 2 d 6. htm Pharm. GKB -- http: //www. pharmgkb. org/
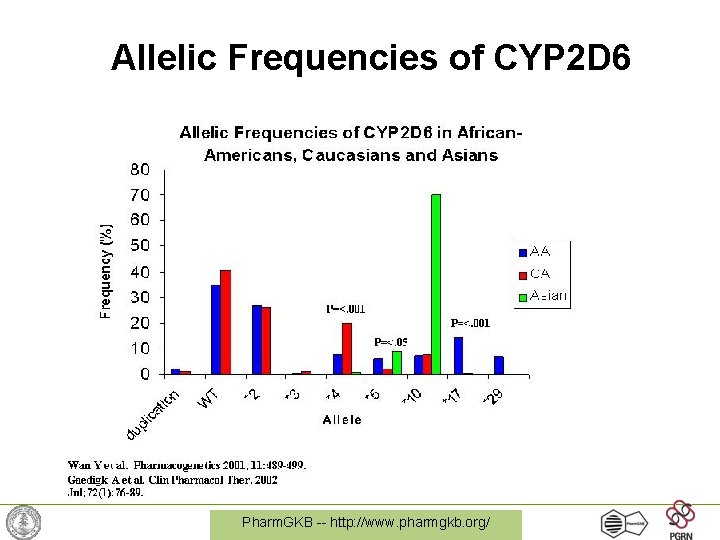
Allelic Frequencies of CYP 2 D 6 Pharm. GKB -- http: //www. pharmgkb. org/

CYP 2 D 6 and Simvastatin • Simvastatin = HMG Co. A reductase, used to decrease LDL, increase HDL cholesterol. • Dose of simvastatin required to get cholesterol-lowering effect is related to 2 D 6 mutations and duplications. Clin Pharmacol Ther. 2001 Dec; 70(6): 546 -51. • Another report demonstrates that “statins” are metabolized differently. Biopharm Drug Dispos. 2000 Dec; 21(9): 353 -64. Pharm. GKB -- http: //www. pharmgkb. org/
Copy number polymorphisms = CNPs • Increasing evidence for variation in the number of copies of a gene in humans • Won’t necessarily be picked up with normal genotyping technology (e. g. sequencing) • Associated with cancers, genetic diseases, and now with drug response variation • Methods for quantifying transcript level, to detect CNPs are coming down in costs Pharm. GKB -- http: //www. pharmgkb. org/
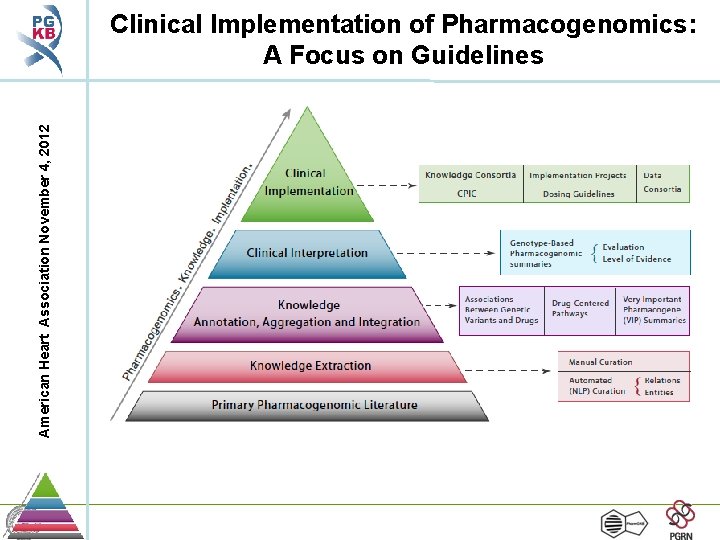
American Heart Association November 4, 2012 Clinical Implementation of Pharmacogenomics: A Focus on Guidelines

Guidelines “We encourage reviewing the primary literature and using one’s clinical judgment rather than relying solely on recommendations. ” Archives Int Med Jan 2011 For the 99. 9% of clinicians who don’t adequately review primary literature, a peer-reviewed group of experts’ review and recommendations would be better.


Survey: Challenges to implementing pharmacogenetics in the clinic What do you think is the most challenging aspect of the implementation of pharmacogenetics into the clinic? A. Translation of genetic information into clinical action B. Test cost, test reimbursement or other economic issues C. Availability of high quality genotyping test (CLIA approved) D. Electronic medical record use, such as the application of CDS E. Clinician and patient resistance and/or ethical concerns Clin Pharmacol Ther. 2011 Mar; 89(3): 464 -7.
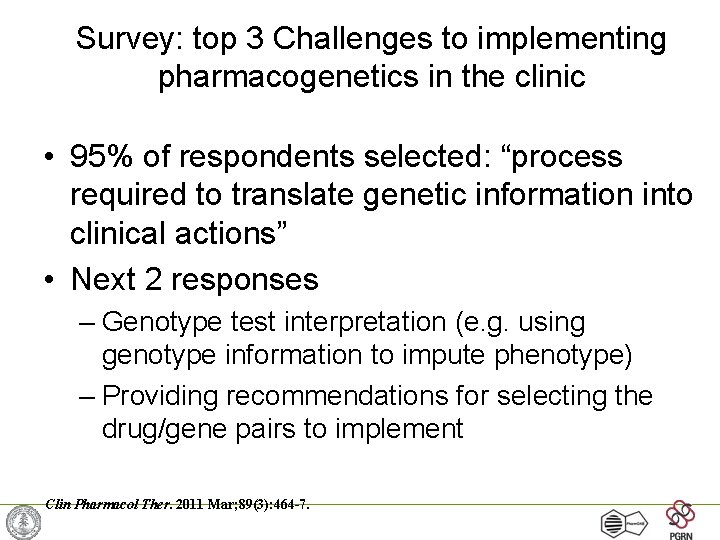
Survey: top 3 Challenges to implementing pharmacogenetics in the clinic • 95% of respondents selected: “process required to translate genetic information into clinical actions” • Next 2 responses – Genotype test interpretation (e. g. using genotype information to impute phenotype) – Providing recommendations for selecting the drug/gene pairs to implement Clin Pharmacol Ther. 2011 Mar; 89(3): 464 -7.
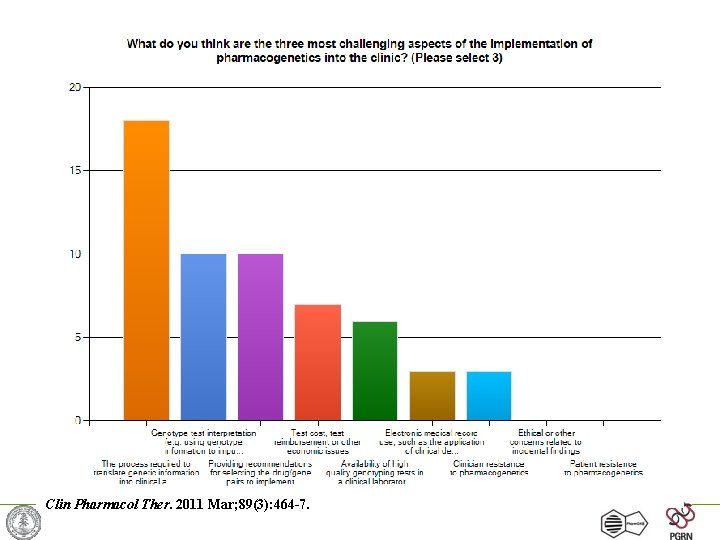
Clin Pharmacol Ther. 2011 Mar; 89(3): 464 -7.

Key Points about a CPIC guideline • Based on assumption that the test results are in hand NOT to discuss the merits of doing the test • Standardized formats • Grading of evidence and of recommendations • Peer reviewed • Freely available • Updated • Authorship with COI policy • Closely follow IOM practices
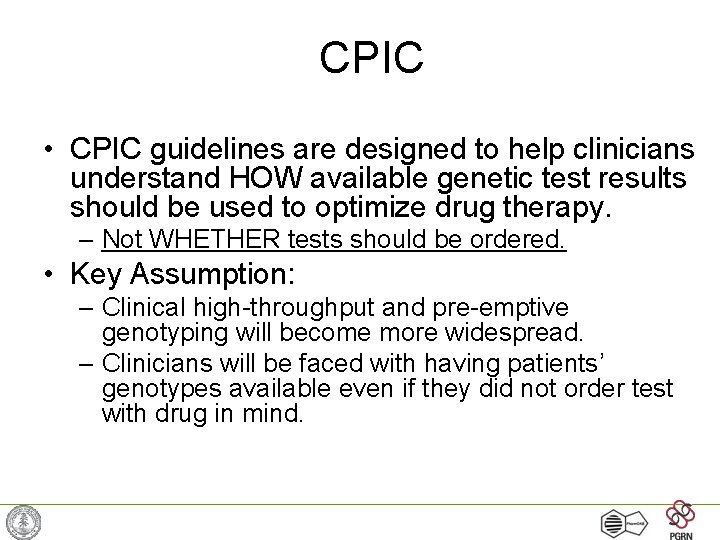
CPIC • CPIC guidelines are designed to help clinicians understand HOW available genetic test results should be used to optimize drug therapy. – Not WHETHER tests should be ordered. • Key Assumption: – Clinical high-throughput and pre-emptive genotyping will become more widespread. – Clinicians will be faced with having patients’ genotypes available even if they did not order test with drug in mind.
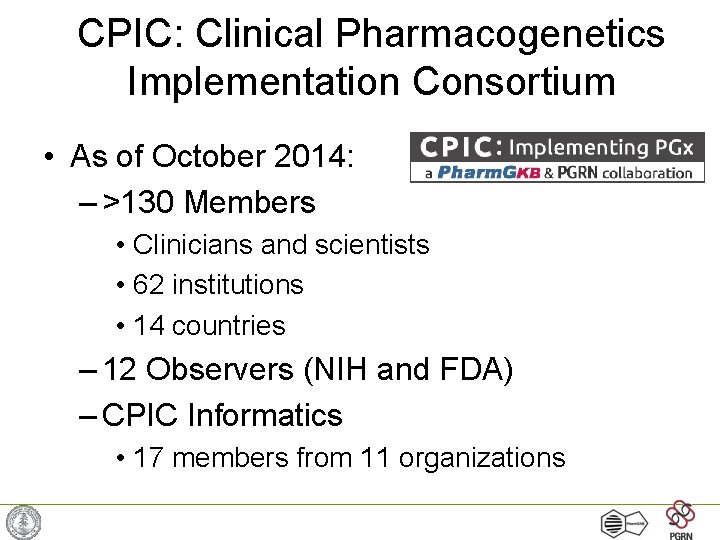
CPIC: Clinical Pharmacogenetics Implementation Consortium • As of October 2014: – >130 Members • Clinicians and scientists • 62 institutions • 14 countries – 12 Observers (NIH and FDA) – CPIC Informatics • 17 members from 11 organizations
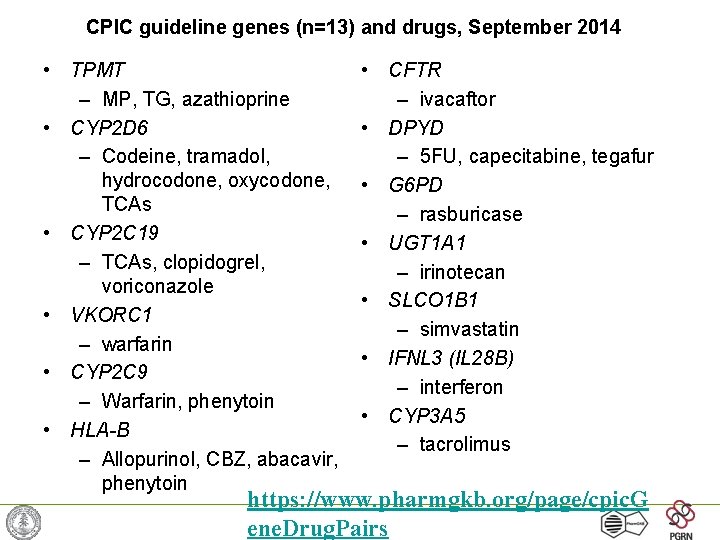
CPIC guideline genes (n=13) and drugs, September 2014 • TPMT – MP, TG, azathioprine • CYP 2 D 6 – Codeine, tramadol, hydrocodone, oxycodone, TCAs • CYP 2 C 19 – TCAs, clopidogrel, voriconazole • VKORC 1 – warfarin • CYP 2 C 9 – Warfarin, phenytoin • HLA-B – Allopurinol, CBZ, abacavir, phenytoin • CFTR – ivacaftor • DPYD – 5 FU, capecitabine, tegafur • G 6 PD – rasburicase • UGT 1 A 1 – irinotecan • SLCO 1 B 1 – simvastatin • IFNL 3 (IL 28 B) – interferon • CYP 3 A 5 – tacrolimus https: //www. pharmgkb. org/page/cpic. G ene. Drug. Pairs
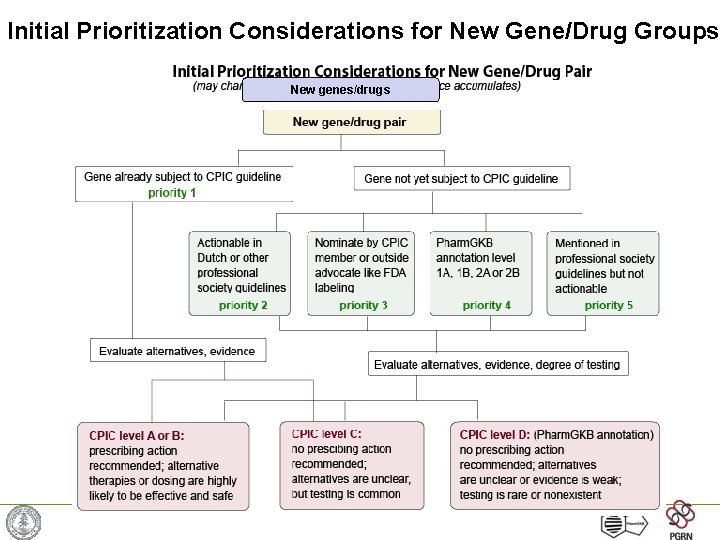
Initial Prioritization Considerations for New Gene/Drug Groups New genes/drugs
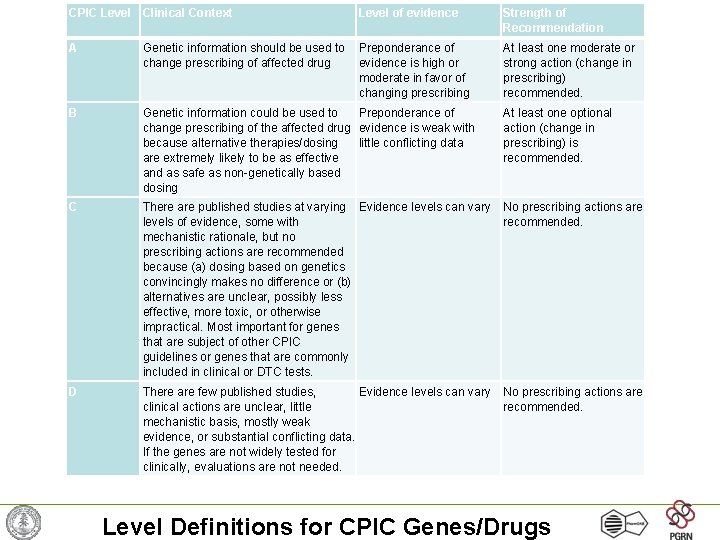
CPIC Level Clinical Context Level of evidence Strength of Recommendation A Genetic information should be used to change prescribing of affected drug Preponderance of evidence is high or moderate in favor of changing prescribing At least one moderate or strong action (change in prescribing) recommended. B Genetic information could be used to Preponderance of change prescribing of the affected drug evidence is weak with because alternative therapies/dosing little conflicting data are extremely likely to be as effective and as safe as non-genetically based dosing At least one optional action (change in prescribing) is recommended. C There are published studies at varying Evidence levels can vary levels of evidence, some with mechanistic rationale, but no prescribing actions are recommended because (a) dosing based on genetics convincingly makes no difference or (b) alternatives are unclear, possibly less effective, more toxic, or otherwise impractical. Most important for genes that are subject of other CPIC guidelines or genes that are commonly included in clinical or DTC tests. No prescribing actions are recommended. D There are few published studies, Evidence levels can vary clinical actions are unclear, little mechanistic basis, mostly weak evidence, or substantial conflicting data. If the genes are not widely tested for clinically, evaluations are not needed. No prescribing actions are recommended. Level Definitions for CPIC Genes/Drugs
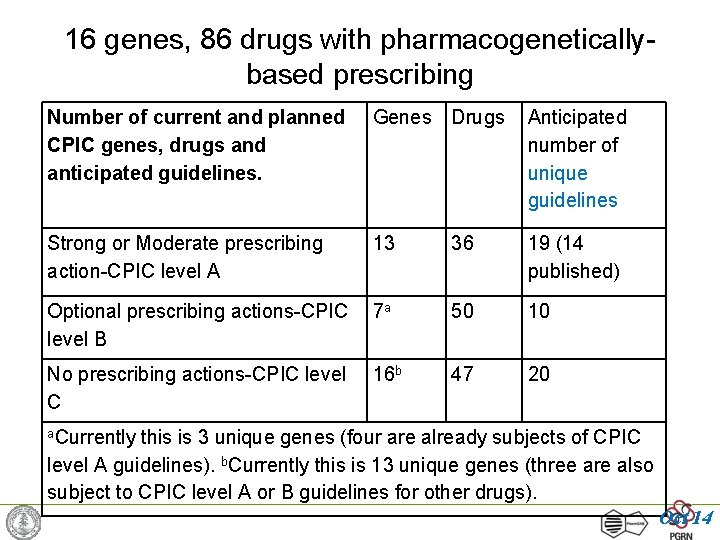
16 genes, 86 drugs with pharmacogeneticallybased prescribing Number of current and planned CPIC genes, drugs and anticipated guidelines. Genes Drugs Anticipated number of unique guidelines Strong or Moderate prescribing action-CPIC level A 13 36 19 (14 published) Optional prescribing actions-CPIC level B 7 a 50 10 No prescribing actions-CPIC level C 16 b 47 20 a. Currently this is 3 unique genes (four are already subjects of CPIC level A guidelines). b. Currently this is 13 unique genes (three are also subject to CPIC level A or B guidelines for other drugs). Oct 14

Clin Pharmacol Ther. 2013 Feb; 93(2): 153 -8 Clin Pharmacol Ther. 2013 May; 93(5): 402 -8. Clin Pharmacol Ther. 2013 Sep; 94(3): 324 -8. Clin Pharmacol Ther. 2013 Apr; 93(4): 324 -5. Clin Pharmacol Ther. 2013 Sep; 94(3): 317 -23 Clin Pharmacol Ther. 2013 Aug 29. Epub Clin Pharmacol Ther. 2014 Feb; 95(2): 141 -6.
CPIC guidelines are posted on Pharm. GKB (www. pharmgkb. org
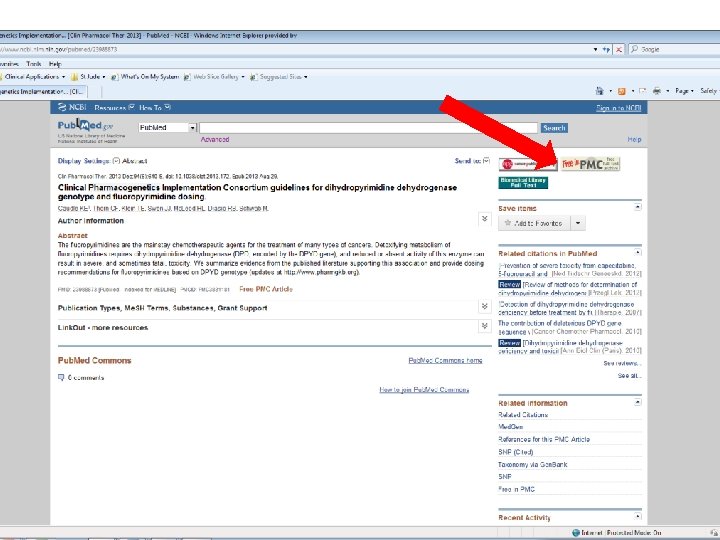
CPIC guidelines linked to “Practice Guideline” filter on Pub. Med
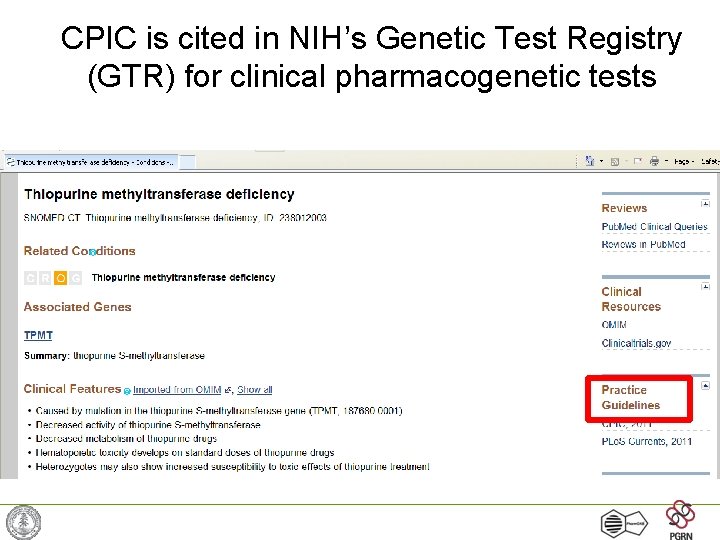
CPIC is cited in NIH’s Genetic Test Registry (GTR) for clinical pharmacogenetic tests

ASHP is endorsing CPIC guidelines

External interactions with other groups • Endorsement by professional societies – ASCPT, ASHP • Continue interactions with www. guidelines. gov, NIH’s GTR, Pub. Med, FDA, NHGRI’s Genomic Medicine Working Group, IOM’s Genomic Medicine Roundtable, PGRN, AMIA, and e. MERGE • Grow interactions with Clin. Gen/Clin. Var

• Purpose: – To describe the development process of the CPIC guidelines – To compare our process to the Institute of Medicine’s Standards for Developing Trustworthy Clinical Practice Guidelines

Caudle et al, Current Drug Me

Uniform Elements of CPIC Guidelines (Main) • Introduction • Focused Literature Review • Gene: – Background – Genetic Test Interpretation • Table 1. Assignment of likely _____ [gene] phenotypes based on genotypes – Available Genetic Test Options – Incidental findings – Other considerations
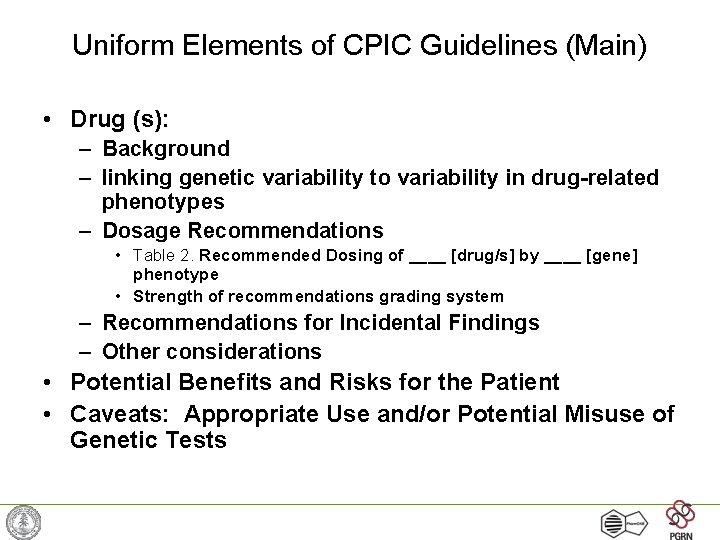
Uniform Elements of CPIC Guidelines (Main) • Drug (s): – Background – linking genetic variability to variability in drug-related phenotypes – Dosage Recommendations • Table 2. Recommended Dosing of ____ [drug/s] by ____ [gene] phenotype • Strength of recommendations grading system – Recommendations for Incidental Findings – Other considerations • Potential Benefits and Risks for the Patient • Caveats: Appropriate Use and/or Potential Misuse of Genetic Tests
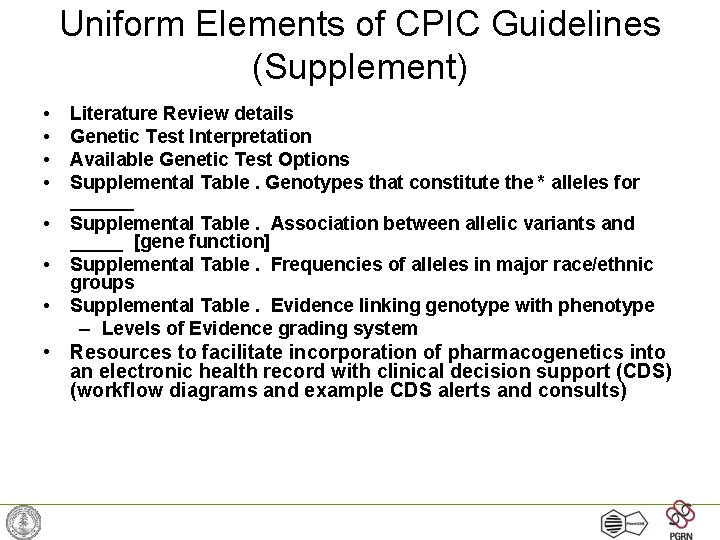
Uniform Elements of CPIC Guidelines (Supplement) • • Literature Review details Genetic Test Interpretation Available Genetic Test Options Supplemental Table. Genotypes that constitute the * alleles for ______ Supplemental Table. Association between allelic variants and _____ [gene function] Supplemental Table. Frequencies of alleles in major race/ethnic groups Supplemental Table. Evidence linking genotype with phenotype – Levels of Evidence grading system • Resources to facilitate incorporation of pharmacogenetics into an electronic health record with clinical decision support (CDS) (workflow diagrams and example CDS alerts and consults)

Clin Pharmacol Ther. 2011
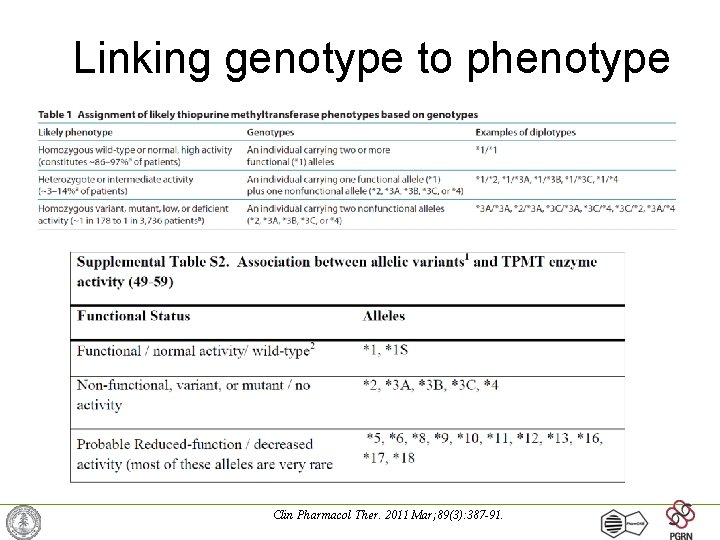
Linking genotype to phenotype Clin Pharmacol Ther. 2011 Mar; 89(3): 387 -91.
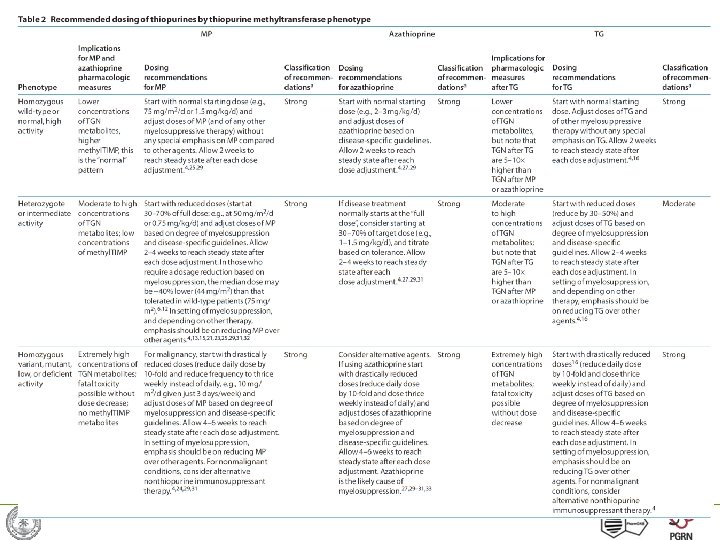

Dosing recommendations: strength based on evidence
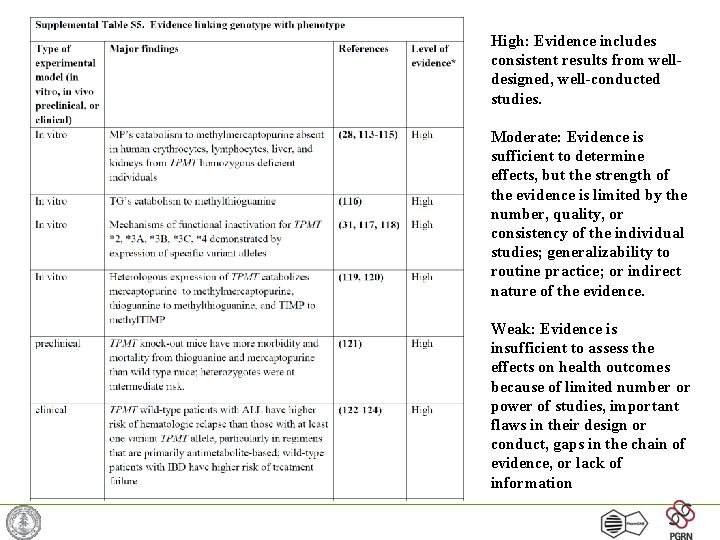
High: Evidence includes consistent results from welldesigned, well-conducted studies. Moderate: Evidence is sufficient to determine effects, but the strength of the evidence is limited by the number, quality, or consistency of the individual studies; generalizability to routine practice; or indirect nature of the evidence. Weak: Evidence is insufficient to assess the effects on health outcomes because of limited number or power of studies, important flaws in their design or conduct, gaps in the chain of evidence, or lack of information

Clin Pharmacol Ther. 2014 Apr; 95(4): 376 -82
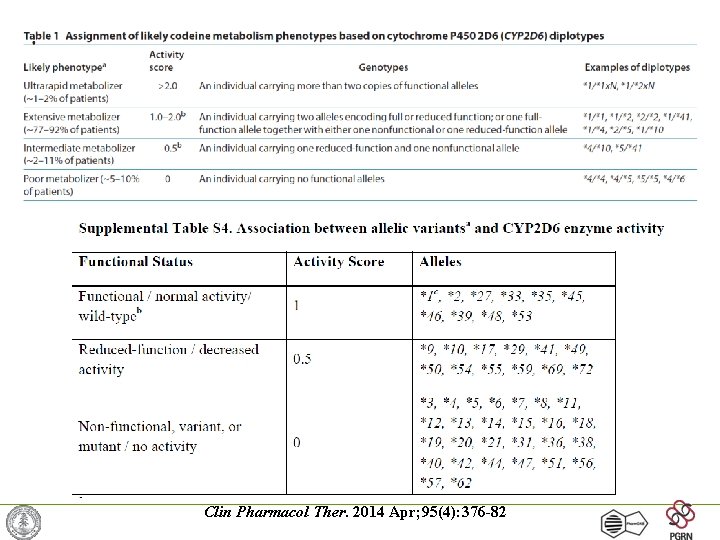
Clin Pharmacol Ther. 2014 Apr; 95(4): 376 -82
Clin Pharmacol Ther. 2012 Apr; 91(4): 734 -8.

Clin Pharmacol Ther. 2012 Jul; 92(1): 112 -7.

CPIC Guidelines Updates • CPIC guidelines are evaluated on an ongoing bases and updated regularly • No change: Update reviewed on Pharm. GKB • Update to publications: – Minor update: Change to supplemental material only – Major update: Changes to both main manuscript and supplement • All changes posted to Pharm. GKB website
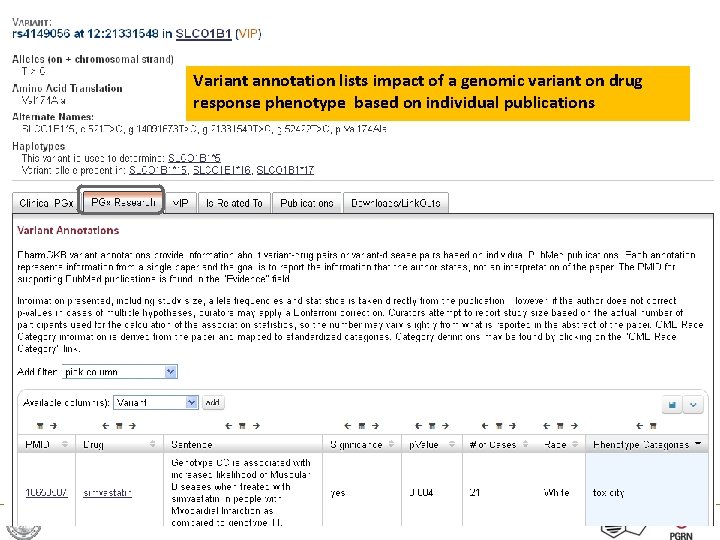
Variant annotation lists impact of a genomic variant on drug response phenotype based on individual publications
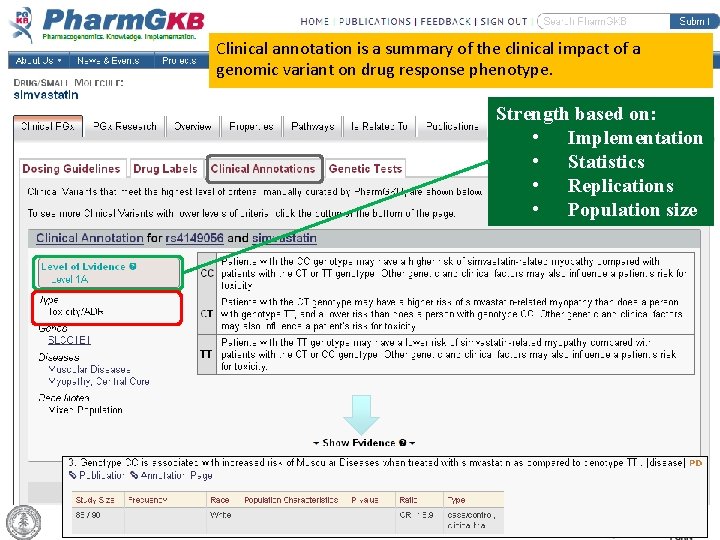
Clinical annotation is a summary of the clinical impact of a genomic variant on drug response phenotype. Strength based on: • Implementation • Statistics • Replications • Population size
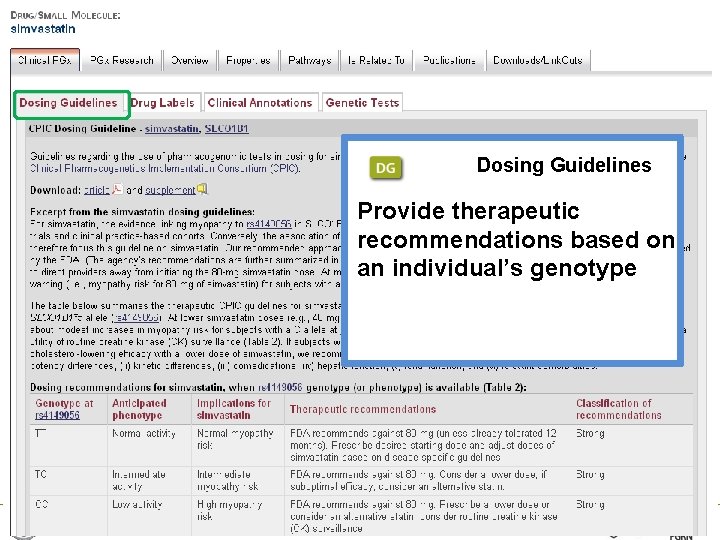
Dosing Guidelines Provide therapeutic recommendations based on an individual’s genotype

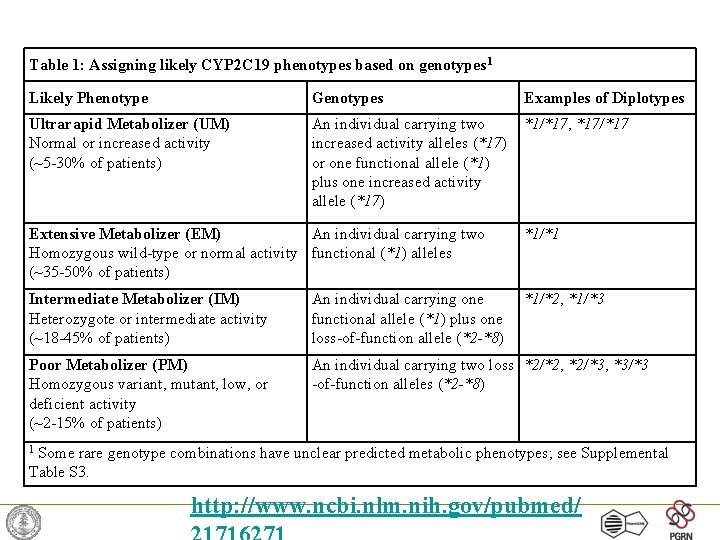
Table 1: Assigning likely CYP 2 C 19 phenotypes based on genotypes 1 Likely Phenotype Genotypes Examples of Diplotypes Ultrarapid Metabolizer (UM) Normal or increased activity (~5 -30% of patients) An individual carrying two increased activity alleles (*17) or one functional allele (*1) plus one increased activity allele (*17) *1/*17, *17/*17 Extensive Metabolizer (EM) An individual carrying two Homozygous wild-type or normal activity functional (*1) alleles (~35 -50% of patients) *1/*1 Intermediate Metabolizer (IM) Heterozygote or intermediate activity (~18 -45% of patients) An individual carrying one functional allele (*1) plus one loss-of-function allele (*2 -*8) *1/*2, *1/*3 Poor Metabolizer (PM) Homozygous variant, mutant, low, or deficient activity (~2 -15% of patients) An individual carrying two loss *2/*2, *2/*3, *3/*3 -of-function alleles (*2 -*8) Some rare genotype combinations have unclear predicted metabolic phenotypes; see Supplemental Table S 3. 1 http: //www. ncbi. nlm. nih. gov/pubmed/
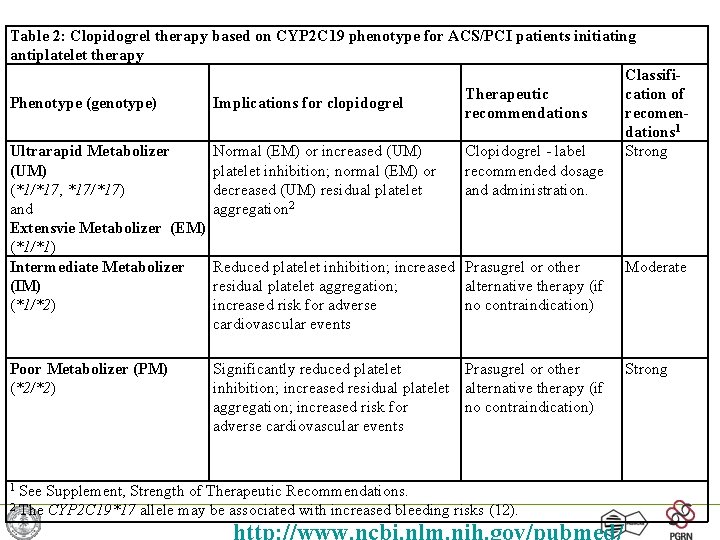
Table 2: Clopidogrel therapy based on CYP 2 C 19 phenotype for ACS/PCI patients initiating antiplatelet therapy Classifi. Therapeutic cation of Phenotype (genotype) Implications for clopidogrel recommendations recomendations 1 Ultrarapid Metabolizer Normal (EM) or increased (UM) Clopidogrel - label Strong (UM) platelet inhibition; normal (EM) or recommended dosage (*1/*17, *17/*17) decreased (UM) residual platelet and administration. and aggregation 2 Extensvie Metabolizer (EM) (*1/*1) Intermediate Metabolizer Reduced platelet inhibition; increased Prasugrel or other Moderate (IM) residual platelet aggregation; alternative therapy (if (*1/*2) increased risk for adverse no contraindication) cardiovascular events Poor Metabolizer (PM) (*2/*2) 1 2 Significantly reduced platelet Prasugrel or other inhibition; increased residual platelet alternative therapy (if aggregation; increased risk for no contraindication) adverse cardiovascular events See Supplement, Strength of Therapeutic Recommendations. The CYP 2 C 19*17 allele may be associated with increased bleeding risks (12). Strong


Drugs for “Patient 0” Genome

PMID: 20435227
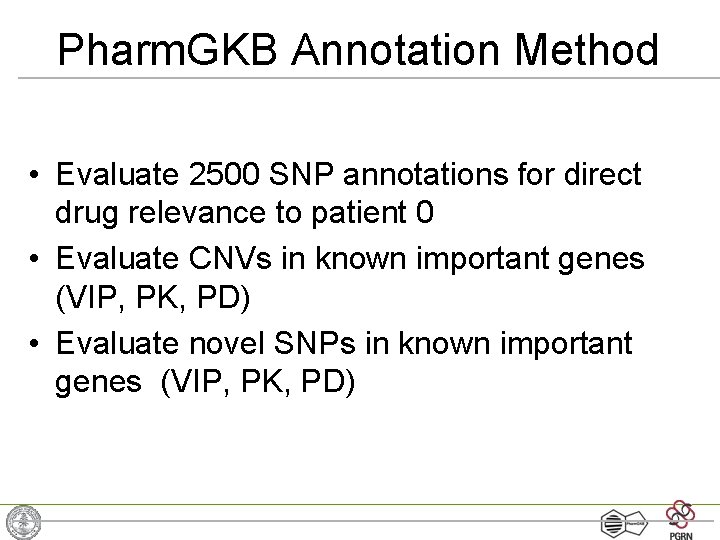
Pharm. GKB Annotation Method • Evaluate 2500 SNP annotations for direct drug relevance to patient 0 • Evaluate CNVs in known important genes (VIP, PK, PD) • Evaluate novel SNPs in known important genes (VIP, PK, PD)
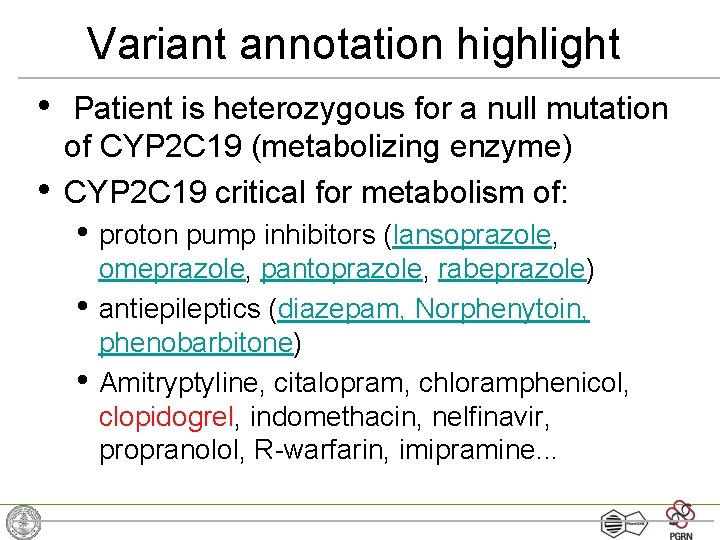
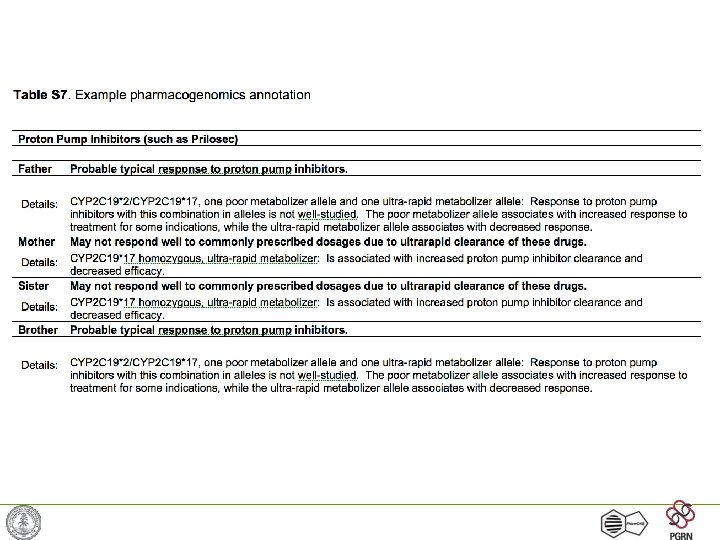
Variant annotation highlight • • Patient is heterozygous for a null mutation of CYP 2 C 19 (metabolizing enzyme) CYP 2 C 19 critical for metabolism of: • proton pump inhibitors (lansoprazole, • • omeprazole, pantoprazole, rabeprazole) antiepileptics (diazepam, Norphenytoin, phenobarbitone) Amitryptyline, citalopram, chloramphenicol, clopidogrel, indomethacin, nelfinavir, propranolol, R-warfarin, imipramine. . .
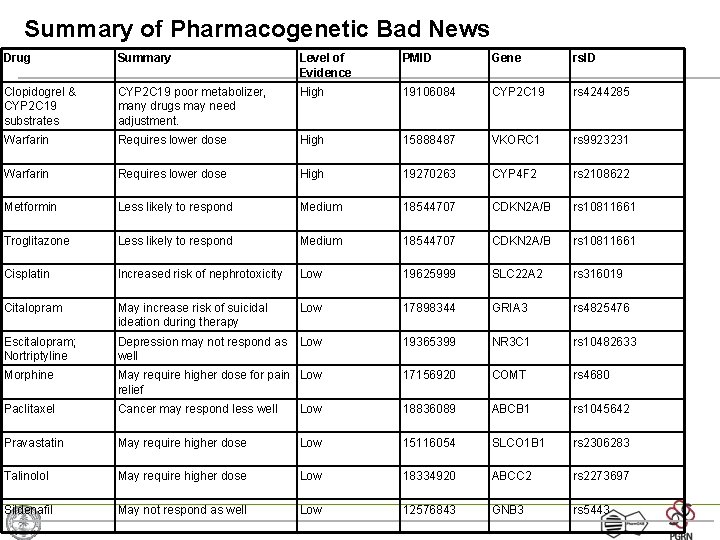
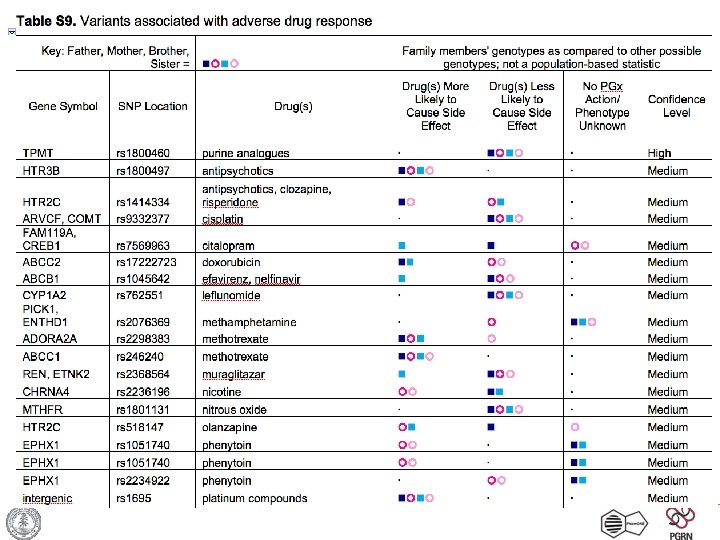
Summary of Pharmacogenetic Bad News Drug Summary Level of Evidence PMID Gene rs. ID Clopidogrel & CYP 2 C 19 substrates CYP 2 C 19 poor metabolizer, many drugs may need adjustment. High 19106084 CYP 2 C 19 rs 4244285 Warfarin Requires lower dose High 15888487 VKORC 1 rs 9923231 Warfarin Requires lower dose High 19270263 CYP 4 F 2 rs 2108622 Metformin Less likely to respond Medium 18544707 CDKN 2 A/B rs 10811661 Troglitazone Less likely to respond Medium 18544707 CDKN 2 A/B rs 10811661 Cisplatin Increased risk of nephrotoxicity Low 19625999 SLC 22 A 2 rs 316019 Citalopram May increase risk of suicidal ideation during therapy Low 17898344 GRIA 3 rs 4825476 Escitalopram; Nortriptyline Depression may not respond as well Low 19365399 NR 3 C 1 rs 10482633 Morphine May require higher dose for pain Low relief 17156920 COMT rs 4680 Paclitaxel Cancer may respond less well Low 18836089 ABCB 1 rs 1045642 Pravastatin May require higher dose Low 15116054 SLCO 1 B 1 rs 2306283 Talinolol May require higher dose Low 18334920 ABCC 2 rs 2273697 Sildenafil May not respond as well Low 12576843 GNB 3 rs 5443
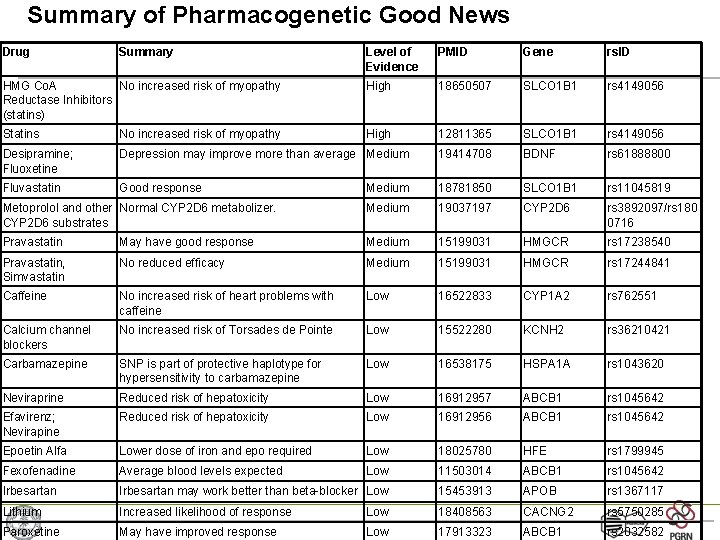
Summary of Pharmacogenetic Good News Drug Summary Level of Evidence PMID Gene rs. ID HMG Co. A No increased risk of myopathy Reductase Inhibitors (statins) High 18650507 SLCO 1 B 1 rs 4149056 Statins No increased risk of myopathy High 12811365 SLCO 1 B 1 rs 4149056 Desipramine; Fluoxetine Depression may improve more than average Medium 19414708 BDNF rs 61888800 Fluvastatin Good response Medium 18781850 SLCO 1 B 1 rs 11045819 Metoprolol and other Normal CYP 2 D 6 metabolizer. CYP 2 D 6 substrates Medium 19037197 CYP 2 D 6 rs 3892097/rs 180 0716 Pravastatin May have good response Medium 15199031 HMGCR rs 17238540 Pravastatin, Simvastatin No reduced efficacy Medium 15199031 HMGCR rs 17244841 Caffeine No increased risk of heart problems with caffeine Low 16522833 CYP 1 A 2 rs 762551 Calcium channel blockers No increased risk of Torsades de Pointe Low 15522280 KCNH 2 rs 36210421 Carbamazepine SNP is part of protective haplotype for hypersensitivity to carbamazepine Low 16538175 HSPA 1 A rs 1043620 Neviraprine Reduced risk of hepatoxicity Low 16912957 ABCB 1 rs 1045642 Efavirenz; Nevirapine Reduced risk of hepatoxicity Low 16912956 ABCB 1 rs 1045642 Epoetin Alfa Lower dose of iron and epo required Low 18025780 HFE rs 1799945 Fexofenadine Average blood levels expected Low 11503014 ABCB 1 rs 1045642 Irbesartan may work better than beta-blocker Low 15453913 APOB rs 1367117 Lithium Increased likelihood of response Low 18408563 CACNG 2 rs 5750285 Paroxetine May have improved response Low 17913323 ABCB 1 rs 2032582
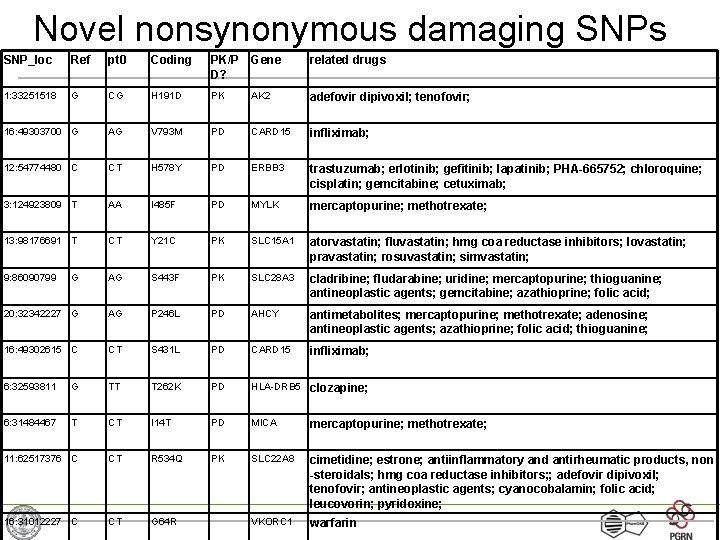
Novel nonsynonymous damaging SNPs SNP_loc Ref pt 0 Coding PK/P D? Gene related drugs 1: 33251518 G CG H 191 D PK AK 2 adefovir dipivoxil; tenofovir; 16: 49303700 G AG V 793 M PD CARD 15 infliximab; 12: 54774480 C CT H 578 Y PD ERBB 3 trastuzumab; erlotinib; gefitinib; lapatinib; PHA-665752; chloroquine; cisplatin; gemcitabine; cetuximab; 3: 124923809 T AA I 485 F PD MYLK mercaptopurine; methotrexate; 13: 98176691 T CT Y 21 C PK SLC 15 A 1 atorvastatin; fluvastatin; hmg coa reductase inhibitors; lovastatin; pravastatin; rosuvastatin; simvastatin; 9: 86090799 G AG S 443 F PK SLC 28 A 3 cladribine; fludarabine; uridine; mercaptopurine; thioguanine; antineoplastic agents; gemcitabine; azathioprine; folic acid; 20: 32342227 G AG P 246 L PD AHCY antimetabolites; mercaptopurine; methotrexate; adenosine; antineoplastic agents; azathioprine; folic acid; thioguanine; 16: 49302615 C CT S 431 L PD CARD 15 infliximab; 6: 32593811 G TT T 262 K PD HLA-DRB 5 clozapine; 6: 31484467 T CT I 14 T PD MICA mercaptopurine; methotrexate; 11: 62517376 C CT R 534 Q PK SLC 22 A 8 cimetidine; estrone; antiinflammatory and antirheumatic products, non -steroidals; hmg coa reductase inhibitors; ; adefovir dipivoxil; tenofovir; antineoplastic agents; cyanocobalamin; folic acid; leucovorin; pyridoxine; 16: 31012227 C CT G 64 R VKORC 1 warfarin

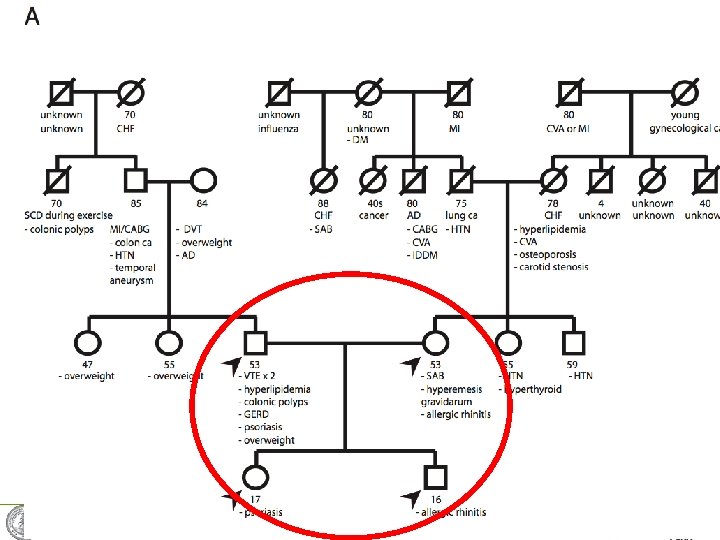
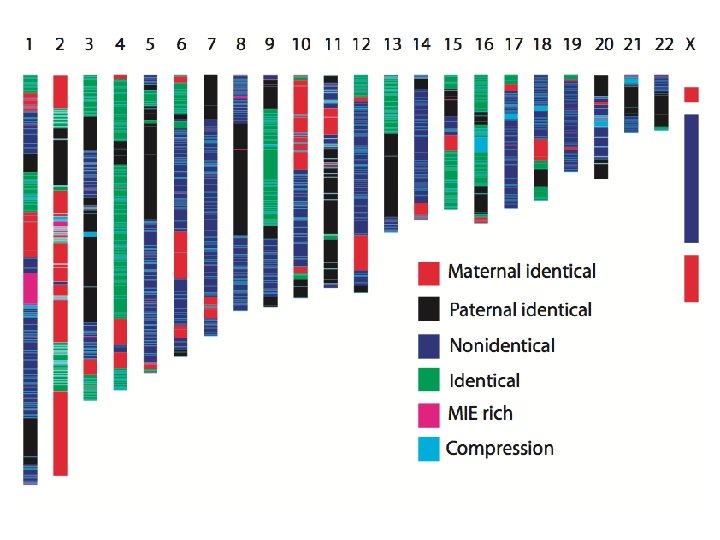
A family quartet PLo. S Genetics, 2011
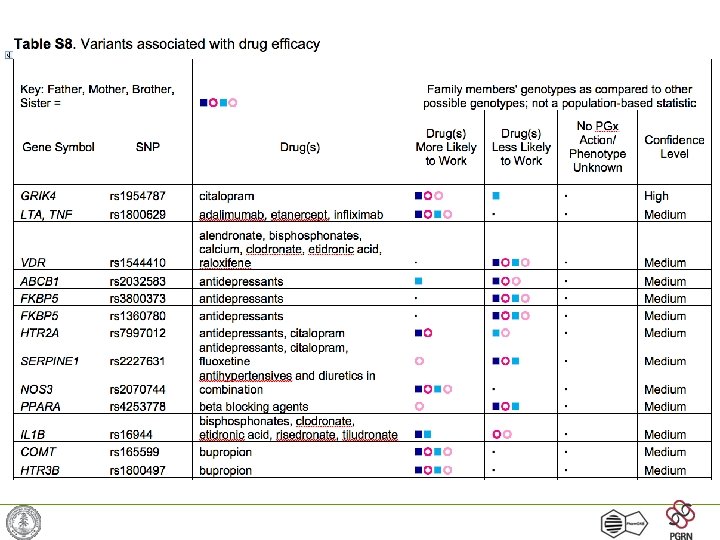
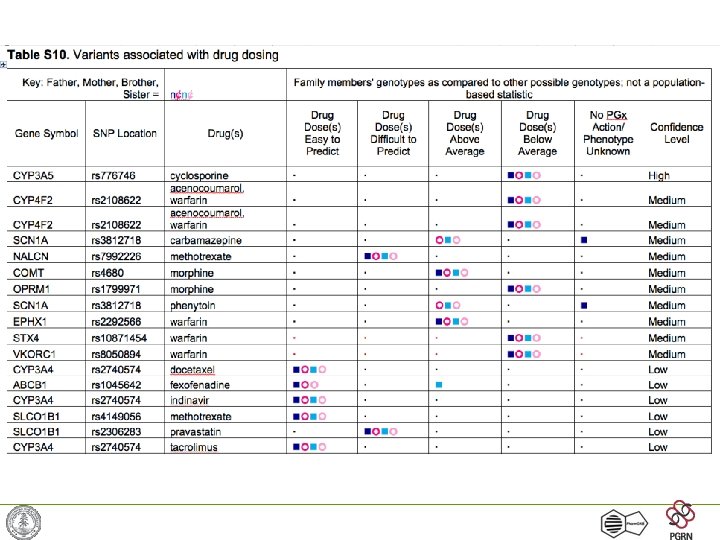

Example: Warfarin dosing • Warfarin used for anticoagulation widely in medicine • Narrow therapeutic index, final dose impossible to predict -> trial and error • Three genes known to affect warfarin dose: CYP 2 C 9, VKORC 1 and CYP 4 F 2 • Recent results suggest warfarin dose will be predicted precisely using genotypes + a few demographic variables Pharm. GKB -- http: //www. pharmgkb. org/

Warfarin Dosing (continued) • Long known: Variation in CYP 2 C 9, but only accounted for < 15% of variation • Found in rat-poison-resistant-rats: Vitamin K epoxide reductase (VKORC 1) • SNP in intron explains 35% of variation! – VKORC 1: -1639 G>A allele • Clinical trials now underway to predict warfarin dose required based on genotype More later on recent developments and implications of warfarin pharmacogenetics Pharm. GKB -- http: //www. pharmgkb. org/

Pharm. GKB -- http: //www. pharmgkb. org/
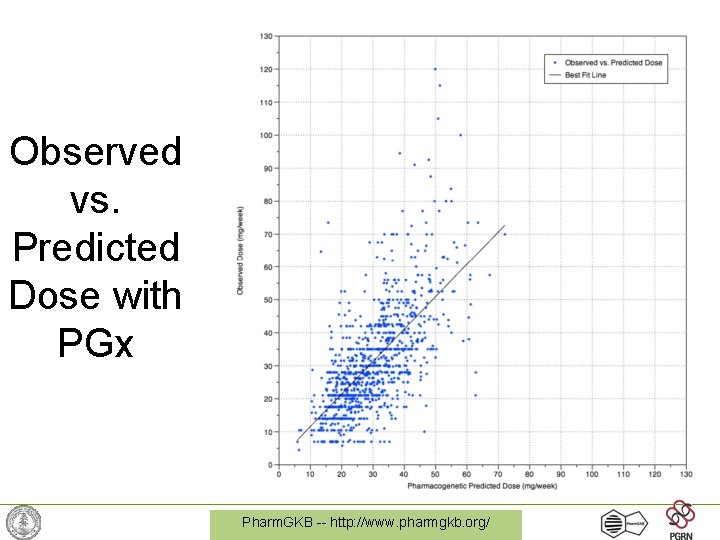
Observed vs. Predicted Dose with PGx Pharm. GKB -- http: //www. pharmgkb. org/
Example: Herceptin & Breast Cancer • HER 2 = human epidermal growth factor • HER 2 is amplified in 25 -35% of breast cancer patients • Herceptin = Trastuzumab = antibody against HER 2 • Herceptin is very effective in those cases, much less effective otherwise • Testing HER 2 levels becomes critical… • Important “First” (survival benefit, ab, test) Pharm. GKB -- http: //www. pharmgkb. org/

Example: Bidil • Combination pill containing two medications for heart failure (hydralazine & isosorbide dinitrate) • Clinical trials did not show overall benefit • Subgroup of African-descent patients showed benefit • Bi. Dil approved for use in African-descent patients Pharm. GKB -- http: //www. pharmgkb. org/
Example: Irinotecan • Powerful anti-neoplastic used in colon/rectal cancers • Use is limited by severe life-threatening diarrhea side effect • Side effect related to patient genotype of UGT 1 A 1 (helps metabolize irinotecan) • Test now marketed for evaluating UGT 1 A 1 genotype prior to initiation of treatment Pharm. GKB -- http: //www. pharmgkb. org/

Pharm. GKB -- http: //www. pharmgkb. org/

Most common major drug ADRs • QT prolongation (heart problems) • Liver failure • Severe dermatological rash • Many other minor ADRs – Minor rash – Abdominal discomfort – Dry mouth – Drowsiness or activation – Headache Pharm. GKB -- http: //www. pharmgkb. org/
Other examples • • Phenylthiourea non-taster phenotype P-glycoprotein transporter variation Aldehyde dehydrogenase CYP 2 C 19 and omeprazole & diazepam Dihydropyridine dehydrogenase and 5 -FU UDP glucuronyl transferase 1 A 1 and bilirubin N-acetylation polymorphism and INH (for TB) NO-sythetase and vascular tone variation… Pharm. GKB -- http: //www. pharmgkb. org/
Example: Gefitinib (Iressa) • Inhibits the growth factor receptor EGFR • Promising early trials - but only improved survival in subset of patients, 10 -15% – Female, – Japanese – Non-smokers Example that shows we have to remember about the host genome as well • Iressa works better in those with variant EGFR that makes it more susceptible to the drug “Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib”, NEJM 2004 350: 2129 -2139 Pharm. GKB -- http: //www. pharmgkb. org/
Hypertension • Beta-adrenergic receptor polymorphism at amino acid 389 (Arg -> Gly) • Less susceptible to beta-blockers (atenolol, metorprolol, propranolol, etc…) • 9 mm Hg vs. 2 mm Hg response to atenolol • May suggest need for testing before giving certain types of anti-hypertensives Pharm. GKB -- http: //www. pharmgkb. org/
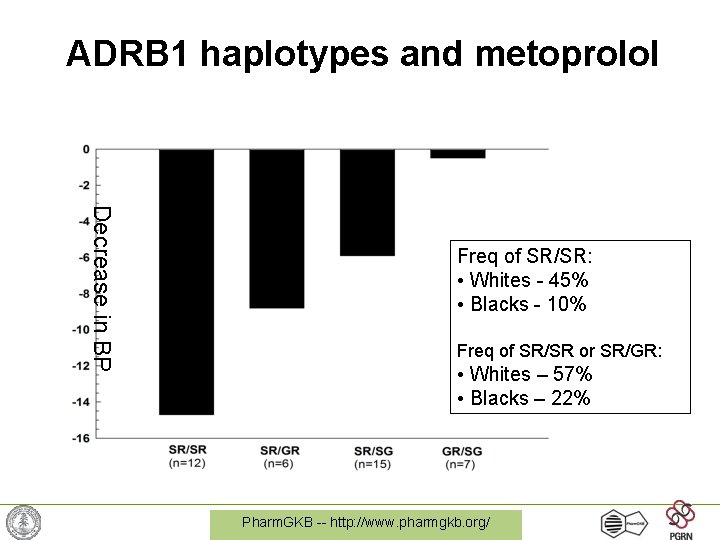
ADRB 1 haplotypes and metoprolol Decrease in BP Freq of SR/SR: • Whites - 45% • Blacks - 10% Freq of SR/SR or SR/GR: • Whites – 57% • Blacks – 22% p = 0. 006 between groups Pharm. GKB -- http: //www. pharmgkb. org/
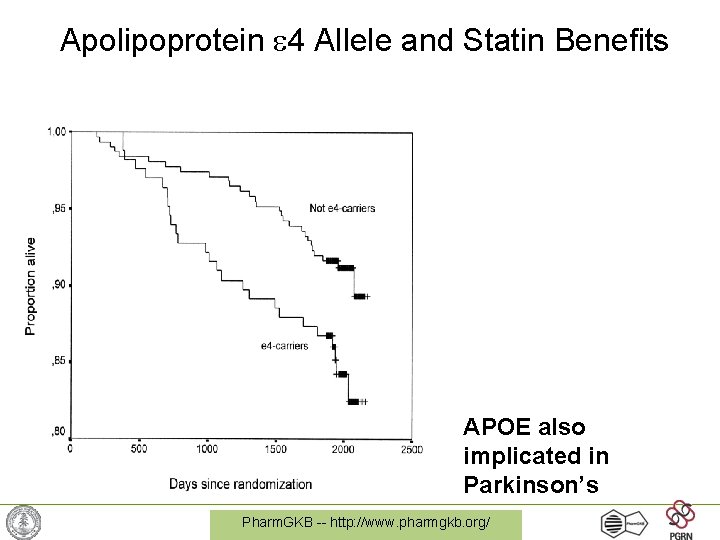
Apolipoprotein 4 Allele and Statin Benefits APOE also implicated in Parkinson’s Pharm. GKB -- http: //www. pharmgkb. org/
Arrhythmia • QT prolongation and arrhythmia risk is the single commonest cause of drug withdrawal or relabeling in the last decade Wall Street Journal Oct. 28, 1999 Pharm. GKB -- http: //www. pharmgkb. org/
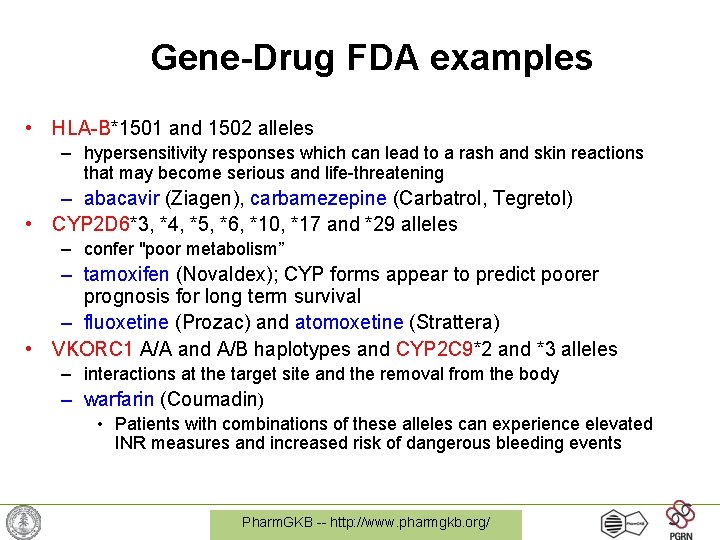
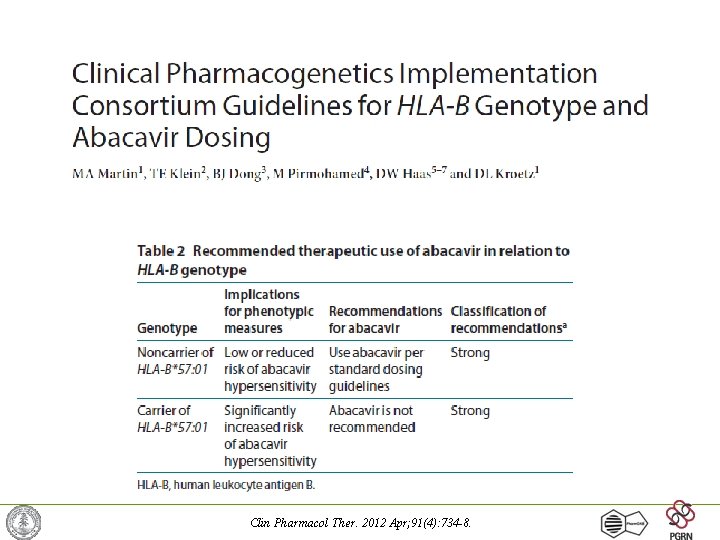
Gene-Drug FDA examples • HLA-B*1501 and 1502 alleles – hypersensitivity responses which can lead to a rash and skin reactions that may become serious and life-threatening – abacavir (Ziagen), carbamezepine (Carbatrol, Tegretol) • CYP 2 D 6*3, *4, *5, *6, *10, *17 and *29 alleles – confer "poor metabolism” – tamoxifen (Novaldex); CYP forms appear to predict poorer prognosis for long term survival – fluoxetine (Prozac) and atomoxetine (Strattera) • VKORC 1 A/A and A/B haplotypes and CYP 2 C 9*2 and *3 alleles – interactions at the target site and the removal from the body – warfarin (Coumadin) • Patients with combinations of these alleles can experience elevated INR measures and increased risk of dangerous bleeding events Pharm. GKB -- http: //www. pharmgkb. org/
Gene-Drugs FDA Examples • UGT 1 A 1*28 allele – reduced clearance of the active form SN-38 of the anticancer drug irinotecan (Camptosar) which can lead to neutropenia and a lifethreatening diarrhea • TPMT*2, *3 A, and *3 C alleles – in homozygous patients leads to decreased clearance – 6 -mercaptopurine (Purinethol) or the pro-drug azothiaprine (Imuran) used to treat acute lymphoblastic leukemia and autoimmune diseases leading to rapid bone marrow suppression that is severe and can become fatal • SLC 01 B 1 (rs 4363657) and OATP 1 B (rs 4149056) – found in liver can determine the blood levels of a wide range of drugs – simvastatin (Zocor) and pravastatin (Pravachol) are associated with development of myopathies • ADRB 2 Arg 16 allele of the beta-2 adrenergic receptor – linked in homozygous patients to a sub-class of asthma and lack of longterm responsiveness to treatment with albuterol (Ventolin) Pharm. GKB -- http: //www. pharmgkb. org/
PGx info in drug labels approved by FDA Frueh et al, Pharmacotherapy 2008 28: 992 -998. • Reviewed labels of FDA approved drugs – to identify those that contained PGx biomarker information – to collect prevalence information on the use of those drugs for which PGx information was included in drug labelling • 1200 drug labels reviewed for 1945 -2005 – 121 drug labels contain PGx info • 69 labels refer to human genomic biomarkers – 62% (43) pertained to cytochrome p 450 s with CYP 2 D 6 most common • 52 labels referred to microbial genomic biomarkers • 24. 3% of prescriptions in 2006 (8. 8 million out of 36. 1 million) processed by a large pharmacy benefits manager received one or more drugs with human genomic biomarker information in the drug label. Pharm. GKB -- http: //www. pharmgkb. org/
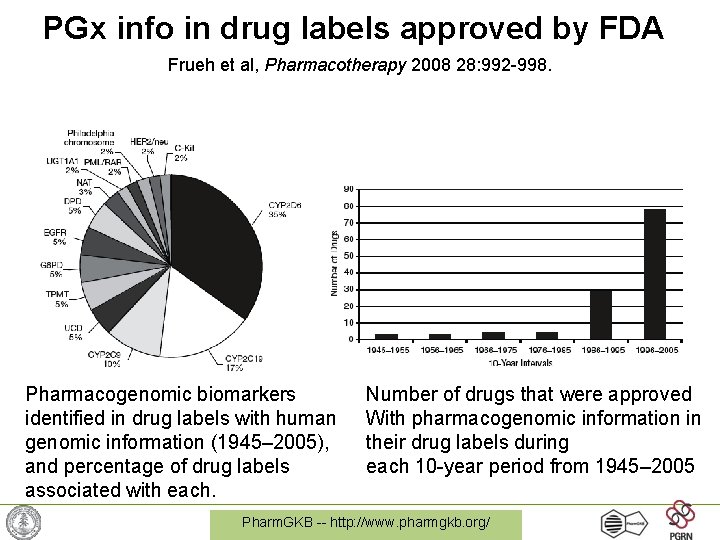
PGx info in drug labels approved by FDA Frueh et al, Pharmacotherapy 2008 28: 992 -998. Pharmacogenomic biomarkers identified in drug labels with human genomic information (1945– 2005), and percentage of drug labels associated with each. Number of drugs that were approved With pharmacogenomic information in their drug labels during each 10 -year period from 1945– 2005 Pharm. GKB -- http: //www. pharmgkb. org/

Conclusions from Drug Data 1945 -2005 “Nearly one fourth of all outpatients received one or more drugs that have pharmacogenomic information in the label for that drug. The incorporation and appropriate use of pharmacogenomic information in drug labels should be tested for its ability to improve drug use and safety in the United States. ” Frueh et al, Pharmacotherapy 2008 28: 992 -998 Pharm. GKB -- http: //www. pharmgkb. org/

Table of Valid Genomic Biomarkers in the Context of Approved Drug Labels http: //www. fda. gov/cder/genomics/genomic_biomarkers_table. htm Pharm. GKB -- http: //www. pharmgkb. org/
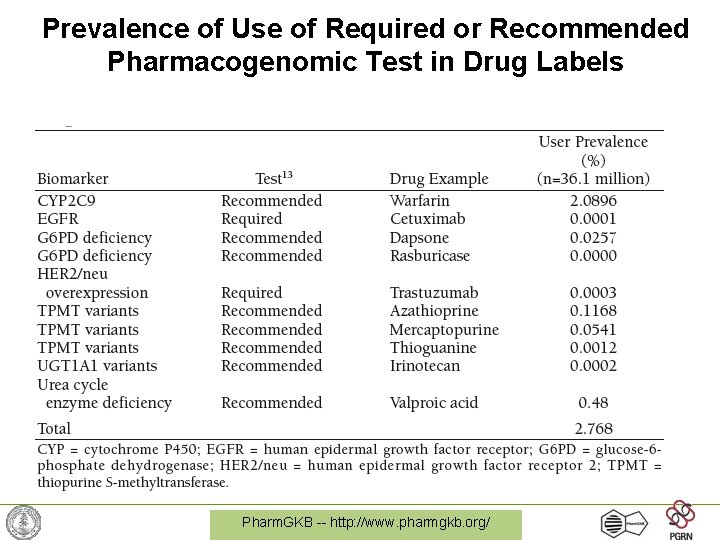
Prevalence of Use of Required or Recommended Pharmacogenomic Test in Drug Labels Pharm. GKB -- http: //www. pharmgkb. org/
http: //medicine. iupui. edu/flockhart/ Pharm. GKB -- http: //www. pharmgkb. org/
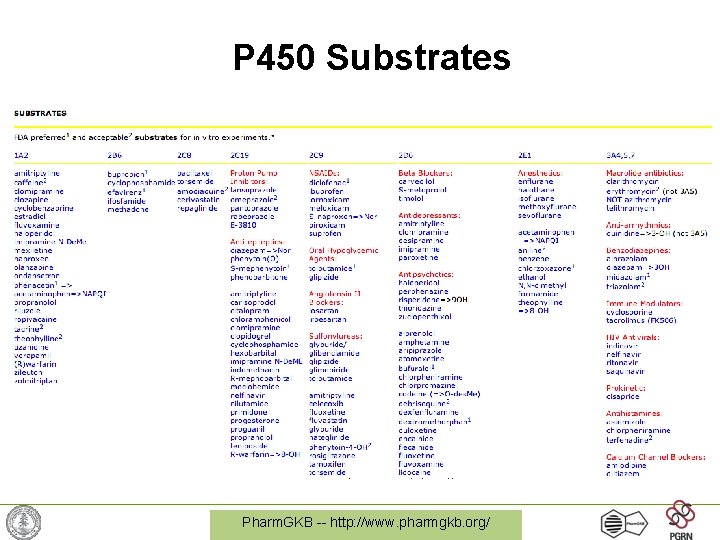
P 450 Substrates Pharm. GKB -- http: //www. pharmgkb. org/

P 450 Inhibitors Pharm. GKB -- http: //www. pharmgkb. org/
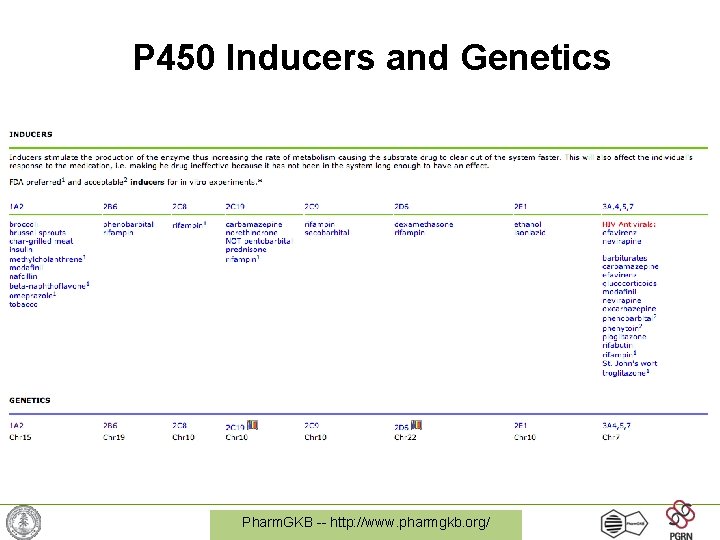
P 450 Inducers and Genetics Pharm. GKB -- http: //www. pharmgkb. org/

Pharm. GKB -- http: //www. pharmgkb. org/

Pharm. GKB -- http: //www. pharmgkb. org/
- Slides: 107