Pharmacoepidemiology Goals and Methods Sean Hennessy Pharm D













- Slides: 26

Pharmacoepidemiology: Goals and Methods Sean Hennessy, Pharm. D, Ph. D Assistant Professor of Epidemiology & Pharmacology Center for Clinical Epidemiology and Biostatistics University of Pennsylvania School of Medicine CCEB

“The study of the use and effects of medications in large numbers of people” Strom

“The application of epidemiologic knowledge, methods, and reasoning to the study of the effects (beneficial and adverse) and use of drugs in human populations. ” Porta and Hartzema

“The study of drugs as determinants of health and disease in the general unselected population. ” Spitzer

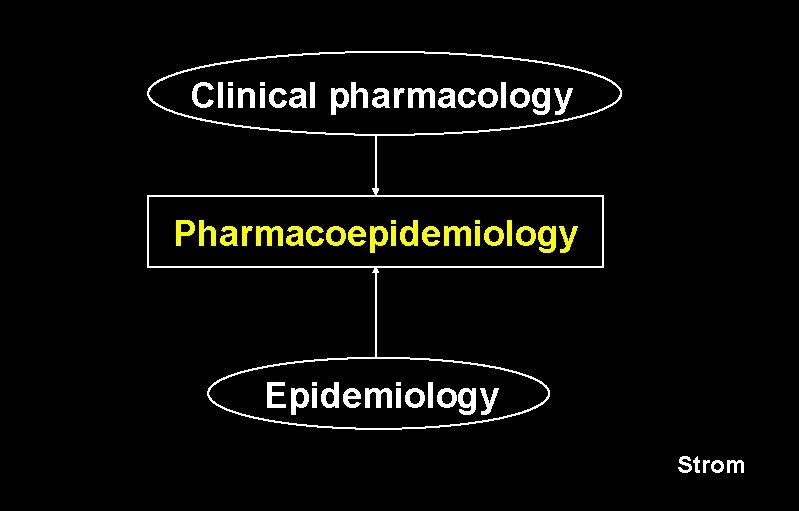
Clinical pharmacology Pharmacoepidemiology Epidemiology Strom

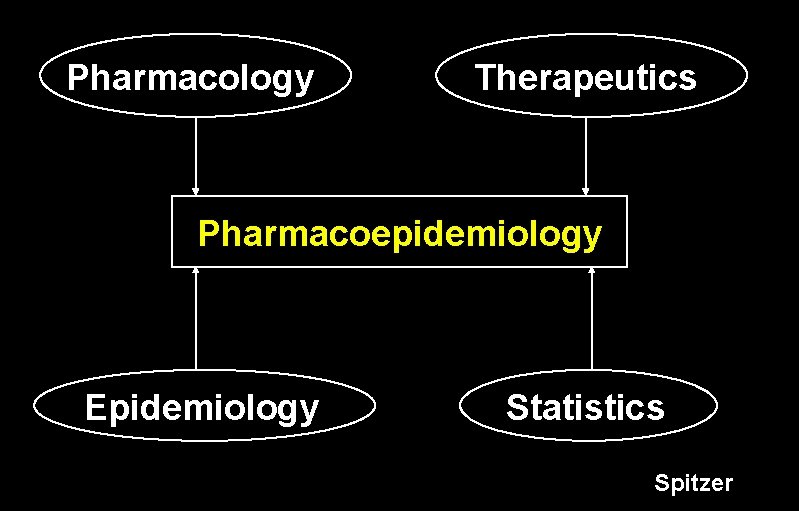
Pharmacology Therapeutics Pharmacoepidemiology Epidemiology Statistics Spitzer

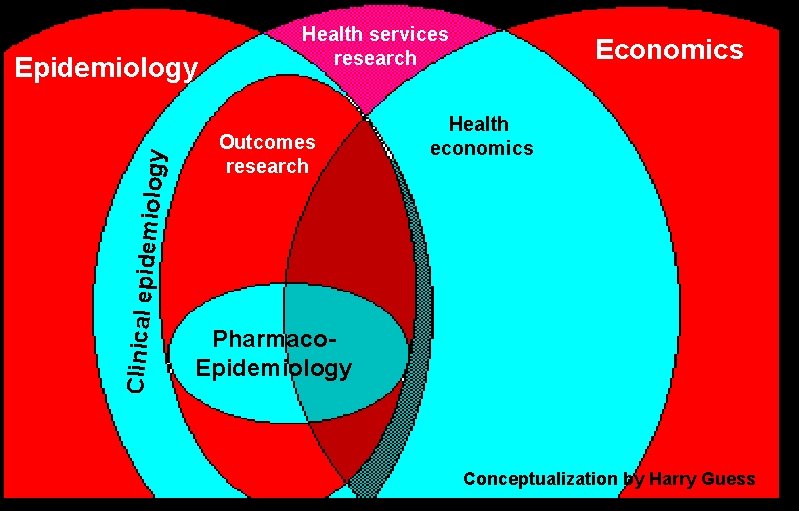
Clinical epid emiology Epidemiology Health services research Outcomes research Economics Health economics Pharmaco. Epidemiology Conceptualization by Harry Guess

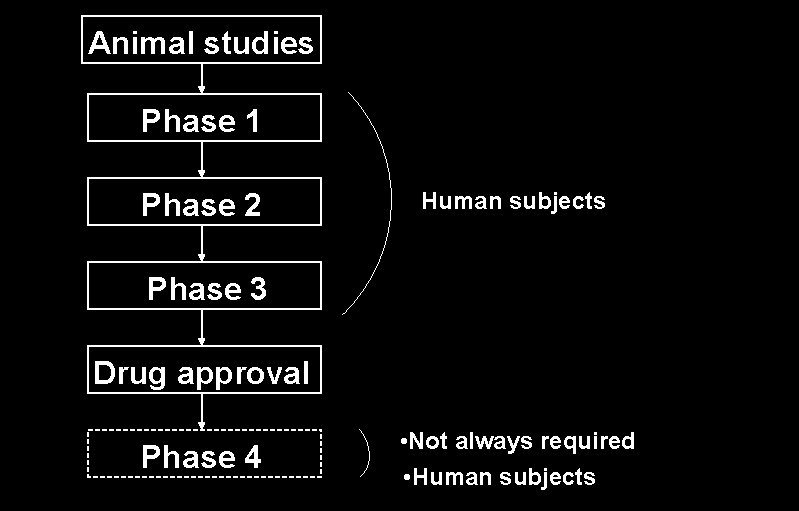
Animal studies Phase 1 Phase 2 Human subjects Phase 3 Drug approval Phase 4 • Not always required • Human subjects

Pre-marketing Post-marketing Pre-de-marketing

Limitations of Pre-marketing Trials-1 • Carefully selected subjects may not reflect real-life patients in whom drug will be used • Study subjects may receive better care than real-life patients • Short duration of treatment

Limitations of pre-marketing trials-2 • Study size –Studies with 3000 patients cannot reliably detect adverse events with an incidence of < 1 per 1000, even if severe –Studies with 500 patients cannot reliably detect adverse events with an incidence of < 1 per 166, even if severe

Consequences of Limitations of Pre-marketing Trials • About 20% of drugs get new “black box” warnings after marketing • About 4% of drugs are ultimately withdrawn for safety reasons


Hypothesis generating Hypothesis strengthening Hypothesis testing

Case Reports & Case Series Cerivastatin (Baycol), an effective and inexpensive lipid lowering drug, was introduced in 1997. It was removed from the market in 2001 because of reports of fatal cases muscle breakdown (rhabdomyolysis).

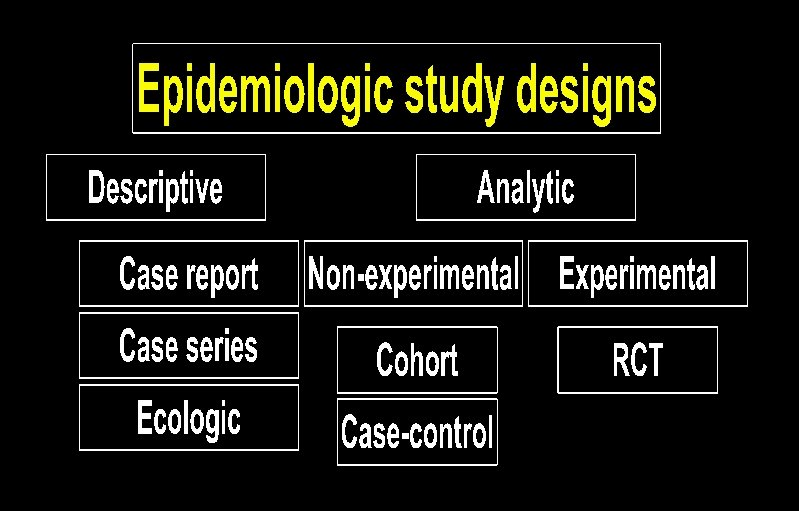
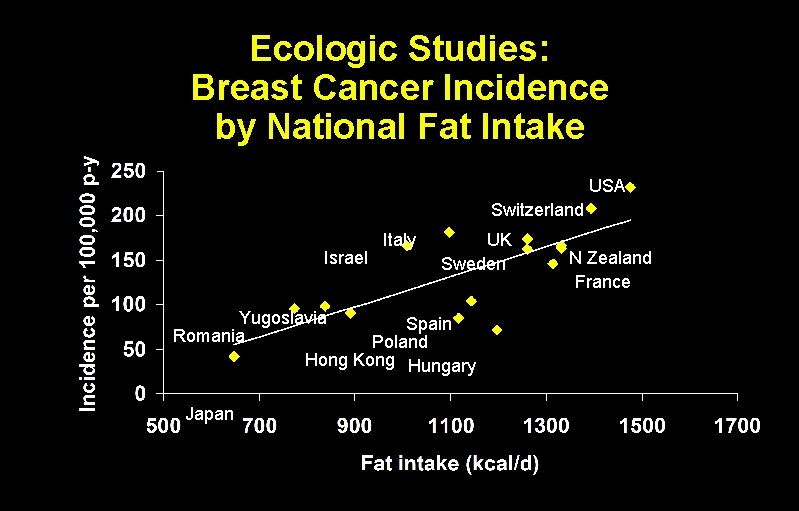
Ecologic studies Obtain group-level exposure information and disease prevalence at the same point in time.

Ecologic Studies: Breast Cancer Incidence by National Fat Intake USA Switzerland Israel Italy UK Sweden Yugoslavia Spain Romania Poland Hong Kong Hungary Japan N Zealand France


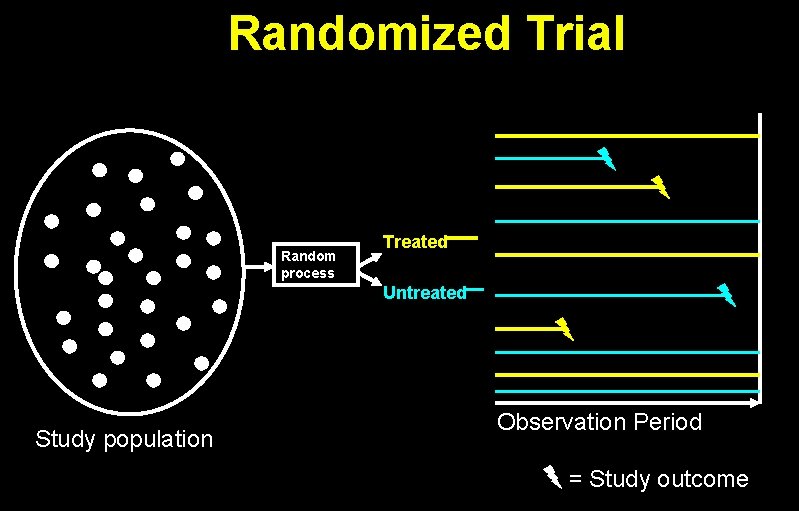
Randomized Trial Random process Treated Untreated Study population Observation Period = Study outcome

Rate Ratio = Events / person-time in exposed Events / person-time in unexposed

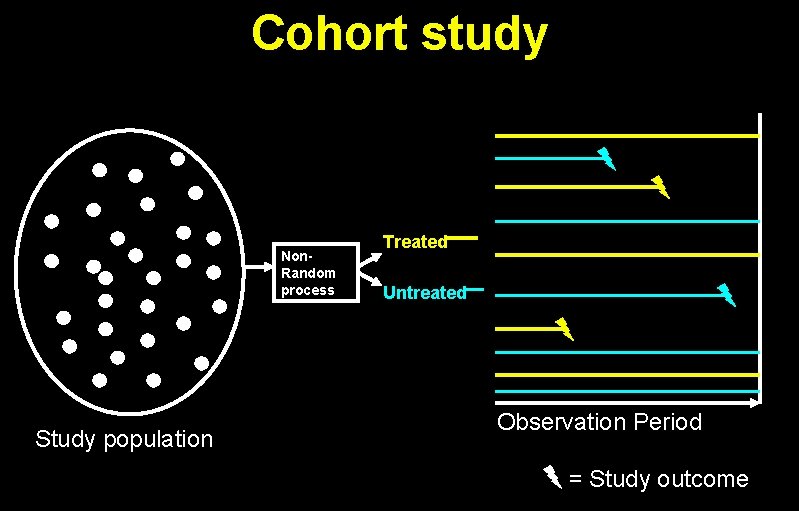
Cohort study Non. Random process Study population Treated Untreated Observation Period = Study outcome

Rate Ratio = Events / person-time in exposed Events / person-time in unexposed A RCT is just a special case of a cohort study

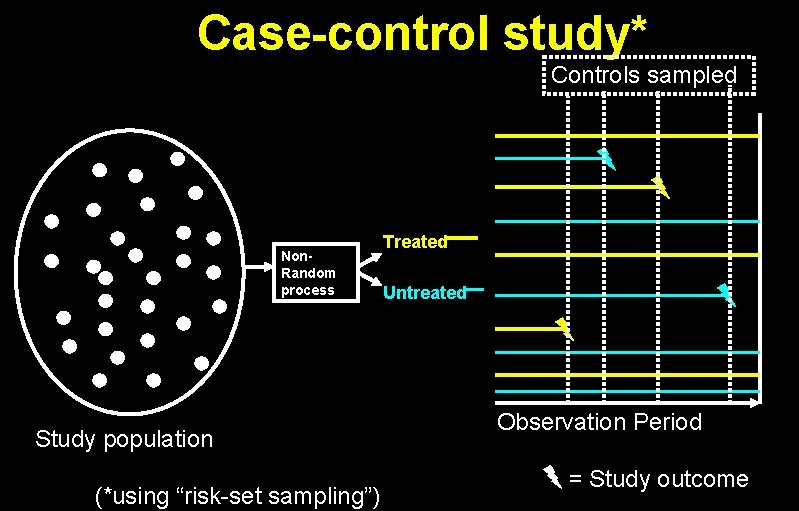
Case-control study* Controls sampled Non. Random process Study population (*using “risk-set sampling”) Treated Untreated Observation Period = Study outcome

Cohort vs. Case-Control • • Advantages of cohort studies -Can calculate incidence -Can directly calculate relative risk -Less measurement error? -Can study multiple outcomes of single exposure Advantages of case-control studies -Need exposure and confounder info on fewer subjects -Often quicker, less expensive -Can study multiple causes of a single disease

Data sources • Spontaneous reporting systems • Ad-hoc studies • Health Care data – Medicaid – General Practice Research Database – Tayside Medicines Monitoring Unit – Group Health Cooperative of Puget Sound – United Health – Etc.
