Pharmacodynamic models 1 Dose response relation PK and

![Emax model n Graphical representations concentrations Log [concentrations] 17 Emax model n Graphical representations concentrations Log [concentrations] 17](https://slidetodoc.com/presentation_image_h/fcb531ad9c9e24dc0ea460efc2345a51/image-16.jpg)
![Emax model n Theoretical basis [L] + [R] [RL] Effect ¨relations KD / EC Emax model n Theoretical basis [L] + [R] [RL] Effect ¨relations KD / EC](https://slidetodoc.com/presentation_image_h/fcb531ad9c9e24dc0ea460efc2345a51/image-17.jpg)
- Slides: 41

Pharmacodynamic models 1

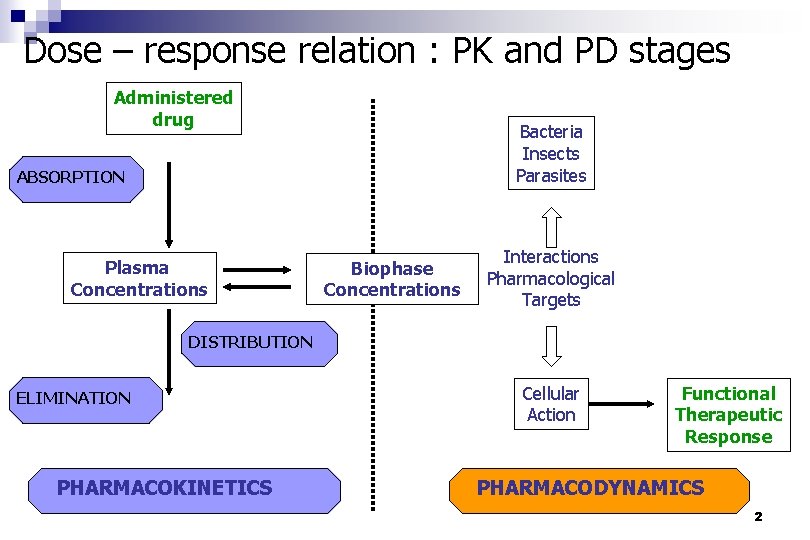
Dose – response relation : PK and PD stages Administered drug Bacteria Insects Parasites ABSORPTION Plasma Concentrations Biophase Concentrations Interactions Pharmacological Targets DISTRIBUTION ELIMINATION PHARMACOKINETICS Cellular Action Functional Therapeutic Response PHARMACODYNAMICS 2

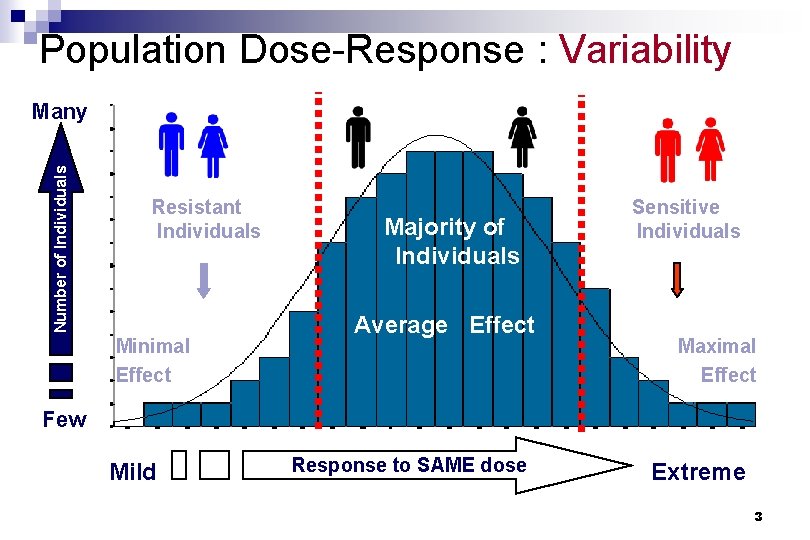
Population Dose-Response : Variability Number of Individuals Many Resistant Individuals Minimal Effect Majority of Individuals Average Effect Sensitive Individuals Maximal Effect Few Mild Response to SAME dose Extreme 3

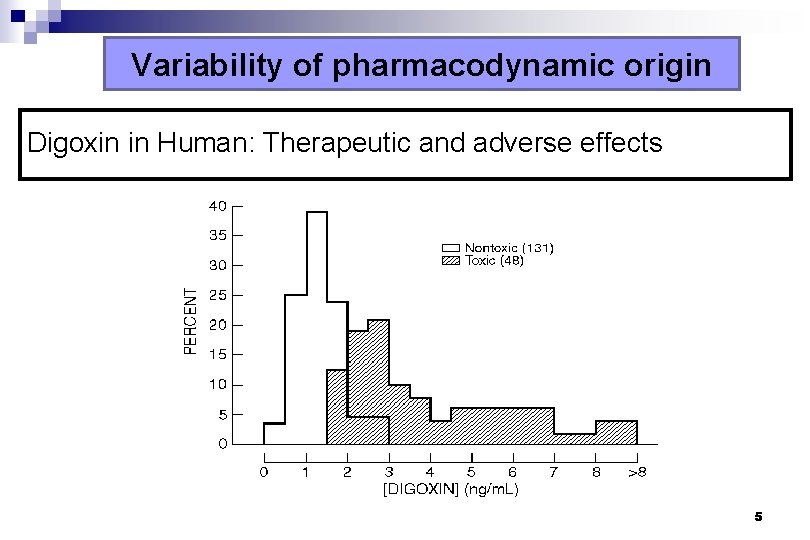
Variability of pharmacodynamic origin Digoxin in Human: Therapeutic and adverse effects 5

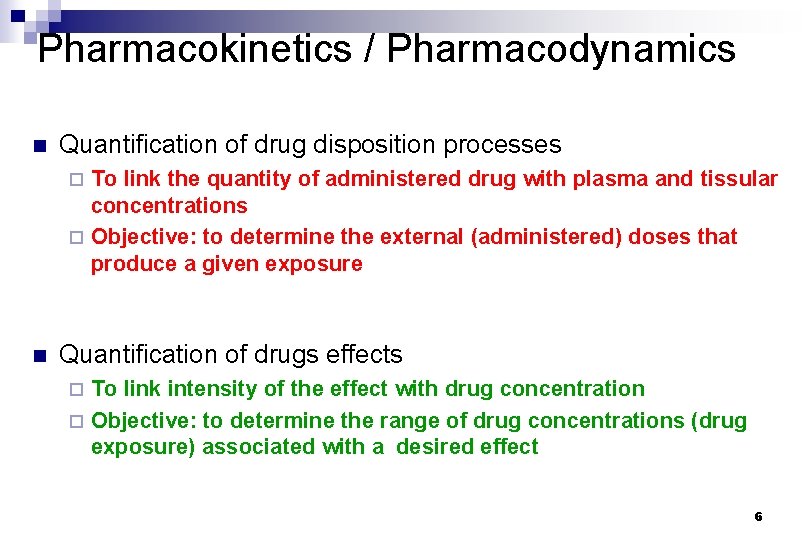
Pharmacokinetics / Pharmacodynamics n Quantification of drug disposition processes To link the quantity of administered drug with plasma and tissular concentrations ¨ Objective: to determine the external (administered) doses that produce a given exposure ¨ n Quantification of drugs effects To link intensity of the effect with drug concentration ¨ Objective: to determine the range of drug concentrations (drug exposure) associated with a desired effect ¨ 6

Effect Endpoints Graded • Continuous scale ( dose ® effect) • Measured in a single biologic unit • Relates dose to intensity of effect Quantal • All-or-none pharmacologic effect • Population studies • Relates dose to frequency of effect 7

n Relation between concentration and the intensity of an effect n Direct effects models n Indirect effects models n Relation between concentration and probability of occurrence of an effect n Fixed-effect model 8

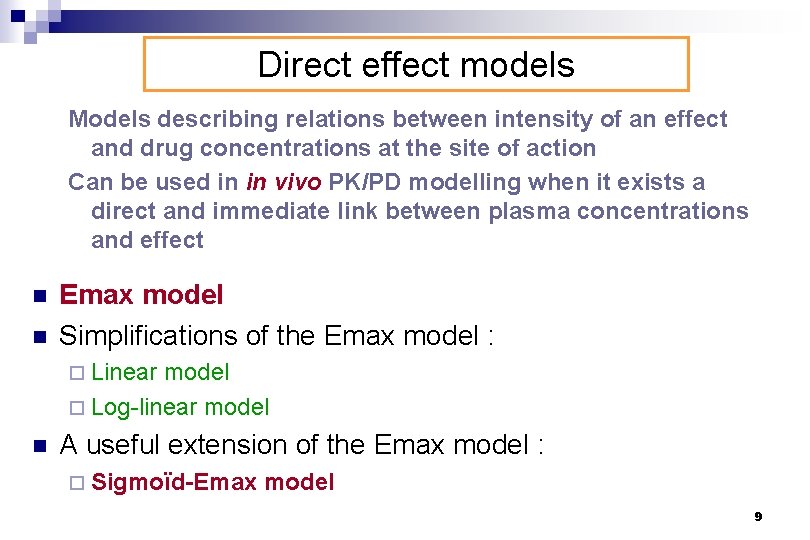
Direct effect models Models describing relations between intensity of an effect and drug concentrations at the site of action Can be used in in vivo PK/PD modelling when it exists a direct and immediate link between plasma concentrations and effect n n Emax model Simplifications of the Emax model : ¨ Linear model ¨ Log-linear model n A useful extension of the Emax model : ¨ Sigmoïd-Emax model 9

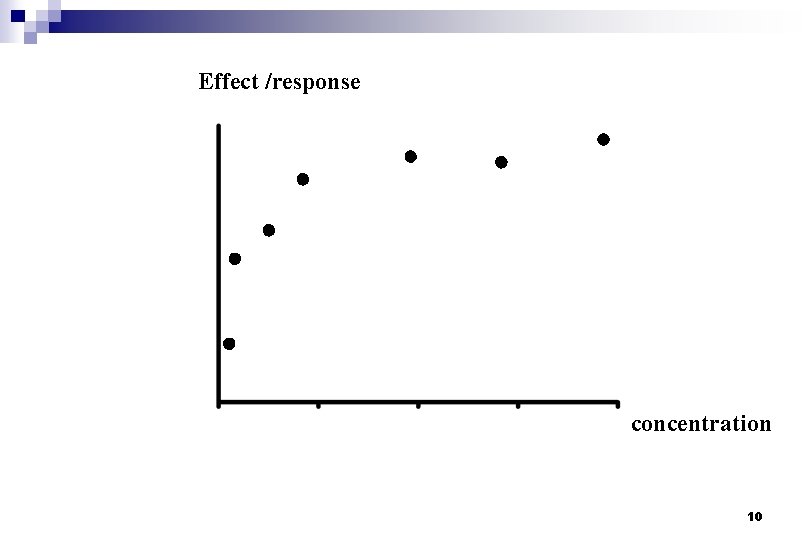
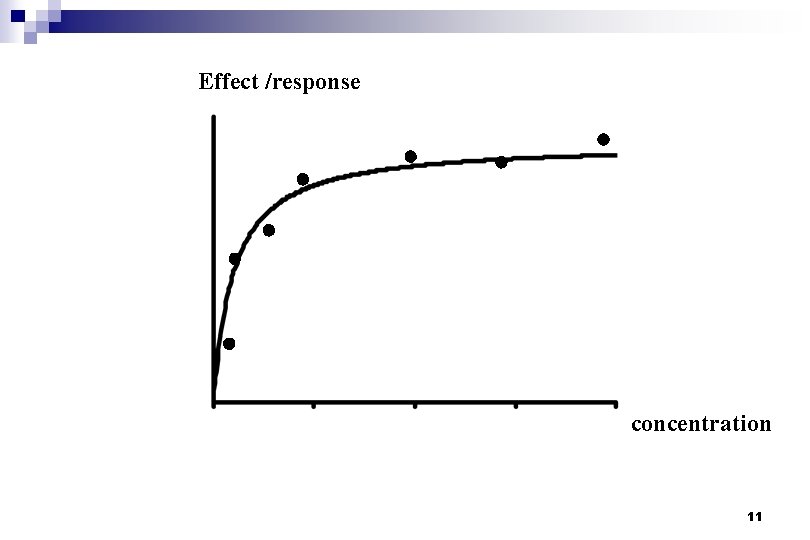
Effect /response concentration 10

Effect /response concentration 11

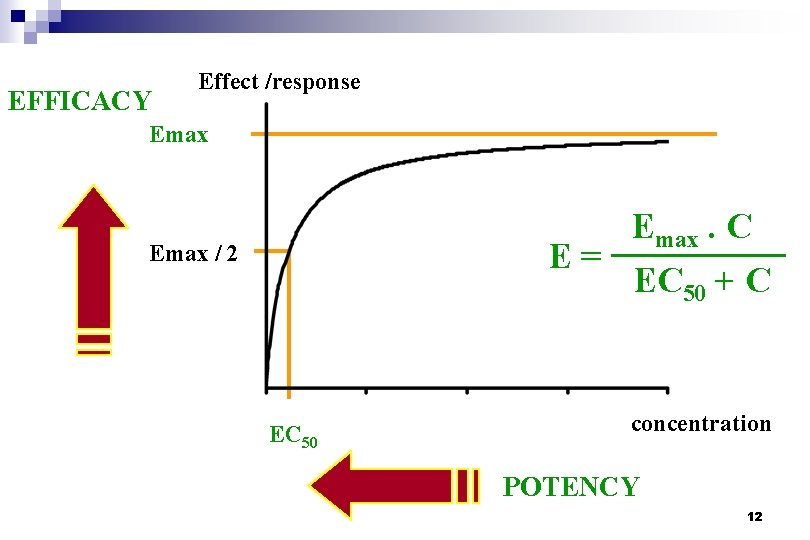
EFFICACY Effect /response Emax. C E= EC 50 + C Emax / 2 EC 50 concentration POTENCY 12

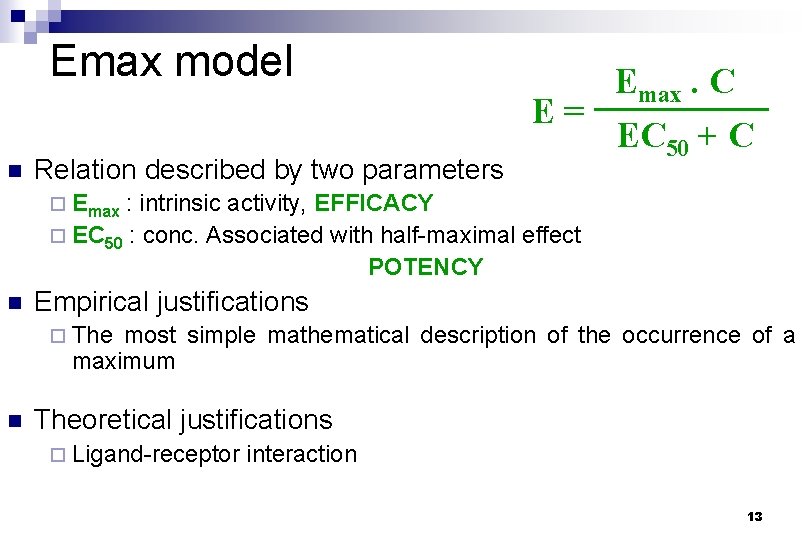
Emax model n Relation described by two parameters Emax. C E= EC 50 + C ¨ Emax : intrinsic activity, EFFICACY ¨ EC 50 : conc. Associated with half-maximal effect POTENCY n Empirical justifications ¨ The most simple mathematical description of the occurrence of a maximum n Theoretical justifications ¨ Ligand-receptor interaction 13

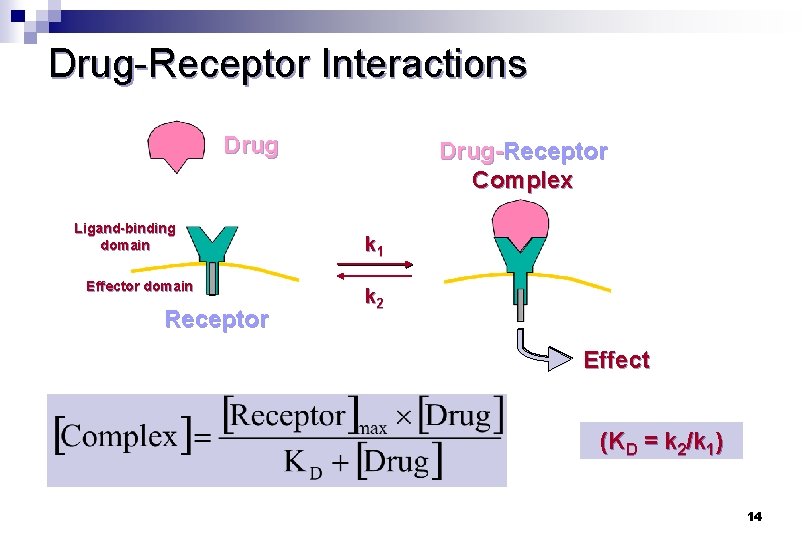
Drug-Receptor Interactions Drug Ligand-binding domain Effector domain Receptor Drug-Receptor Complex k 1 k 2 Effect (KD = k 2/k 1) 14

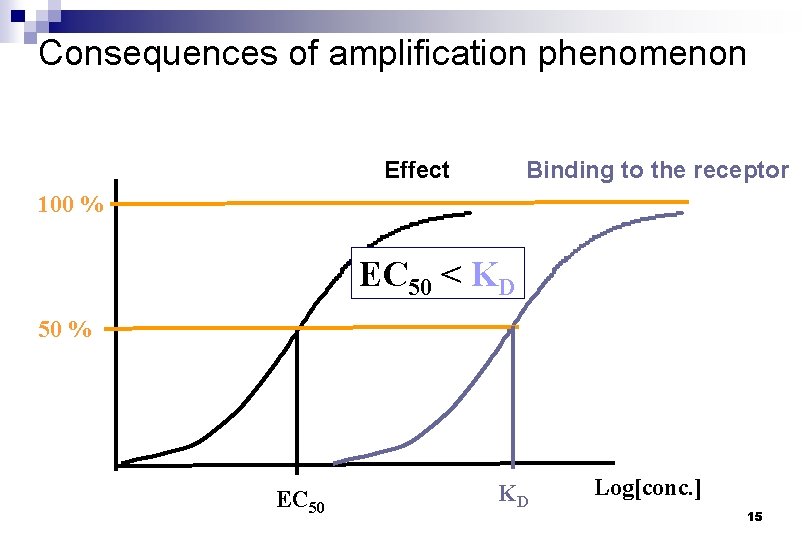
Consequences of amplification phenomenon Effect Binding to the receptor 100 % EC 50 < KD 50 % EC 50 KD Log[conc. ] 15

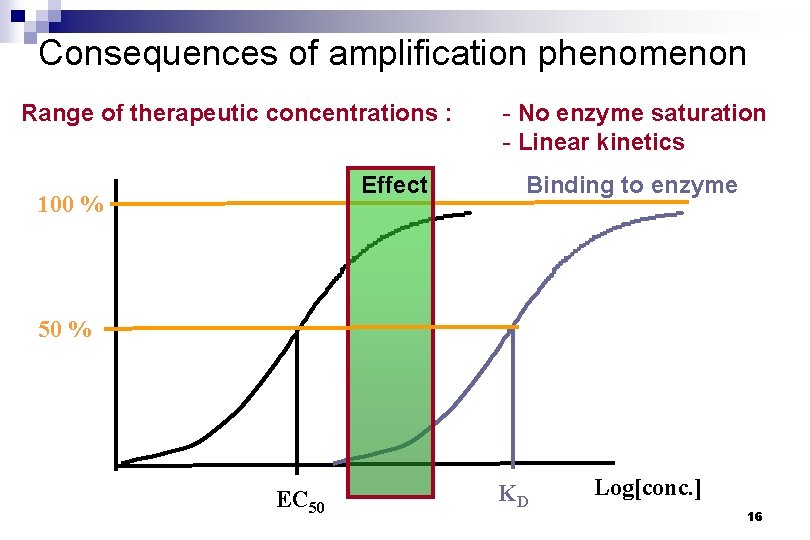
Consequences of amplification phenomenon Range of therapeutic concentrations : Effect 100 % - No enzyme saturation - Linear kinetics Binding to enzyme 50 % EC 50 KD Log[conc. ] 16
![Emax model n Graphical representations concentrations Log concentrations 17 Emax model n Graphical representations concentrations Log [concentrations] 17](https://slidetodoc.com/presentation_image_h/fcb531ad9c9e24dc0ea460efc2345a51/image-16.jpg)
Emax model n Graphical representations concentrations Log [concentrations] 17
![Emax model n Theoretical basis L R RL Effect relations KD EC Emax model n Theoretical basis [L] + [R] [RL] Effect ¨relations KD / EC](https://slidetodoc.com/presentation_image_h/fcb531ad9c9e24dc0ea460efc2345a51/image-17.jpg)
Emax model n Theoretical basis [L] + [R] [RL] Effect ¨relations KD / EC 50 n Graphical representation ¨conc. in arithmetic scale : hyperbola ¨conc. in logarithmic scale : sigmoïd n Comparison potency of drugs in term of efficacy and 18

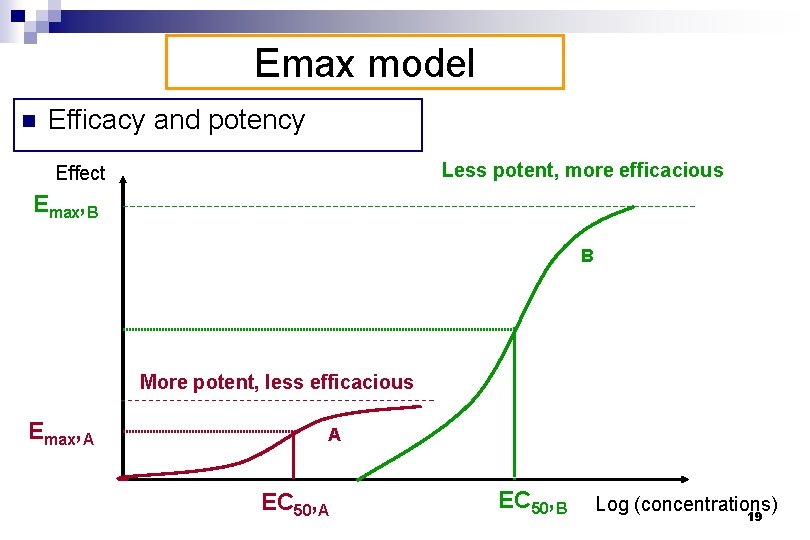
Emax model n Efficacy and potency Less potent, more efficacious Effect Emax, B B More potent, less efficacious Emax, A A EC 50, B Log (concentrations) 19

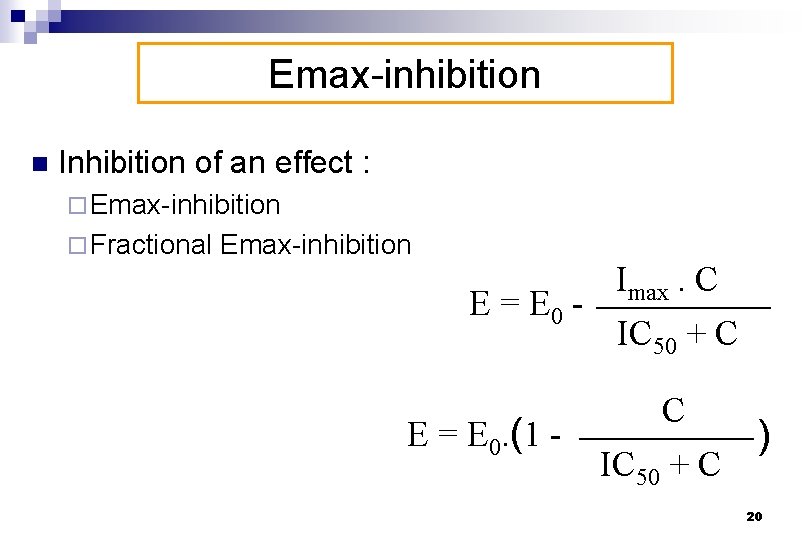
Emax-inhibition n Inhibition of an effect : ¨ Emax-inhibition ¨ Fractional Emax-inhibition Imax. C E = E 0 IC 50 + C E = E 0. (1 - C IC 50 + C ) 20

Simplifications of the Emax model Linear model n Log-linear model n 21

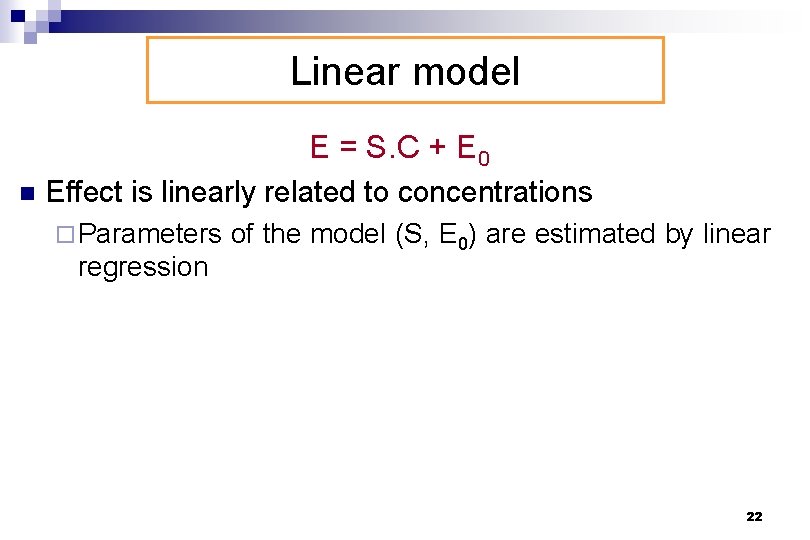
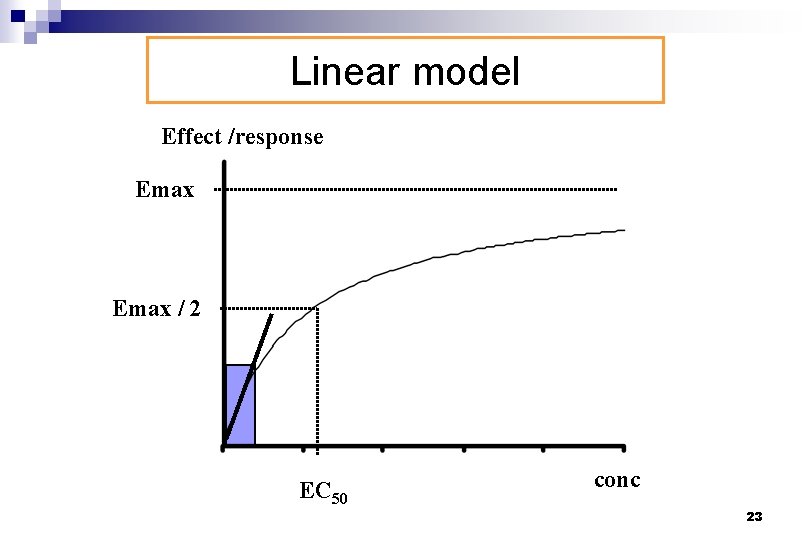
Linear model E = S. C + E 0 n Effect is linearly related to concentrations ¨ Parameters of the model (S, E 0) are estimated by linear regression 22

Linear model Effect /response Emax / 2 EC 50 conc 23

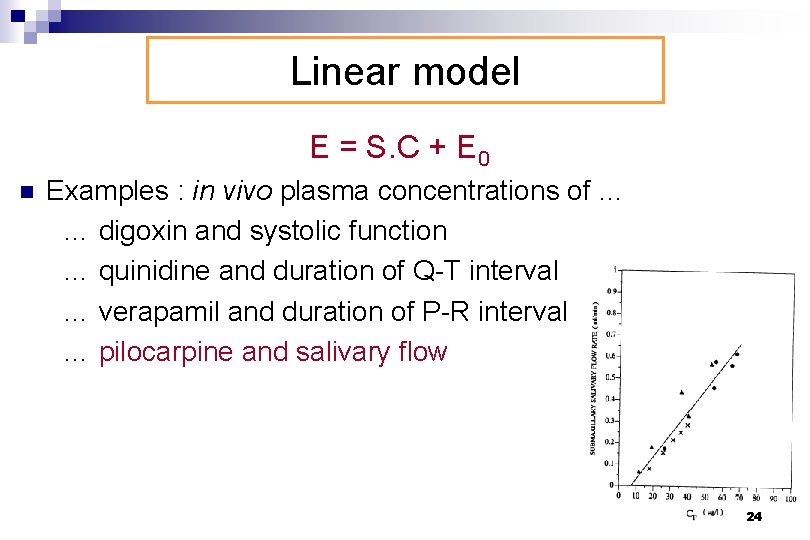
Linear model E = S. C + E 0 n Examples : in vivo plasma concentrations of … … digoxin and systolic function … quinidine and duration of Q-T interval … verapamil and duration of P-R interval … pilocarpine and salivary flow 24

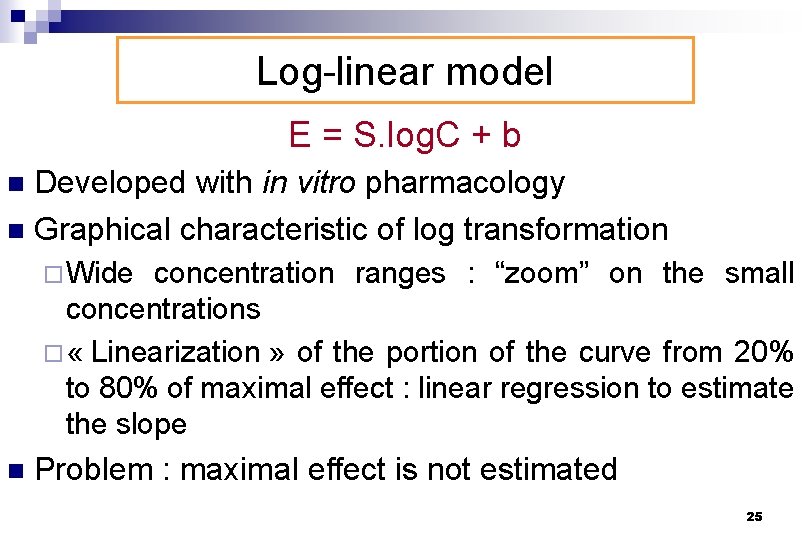
Log-linear model E = S. log. C + b Developed with in vitro pharmacology n Graphical characteristic of log transformation n ¨Wide concentration ranges : “zoom” on the small concentrations ¨ « Linearization » of the portion of the curve from 20% to 80% of maximal effect : linear regression to estimate the slope n Problem : maximal effect is not estimated 25

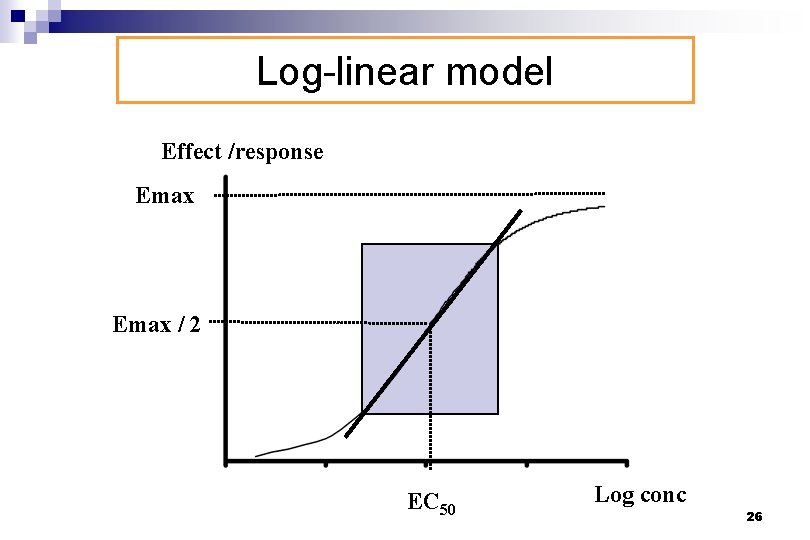
Log-linear model Effect /response Emax / 2 EC 50 Log conc 26

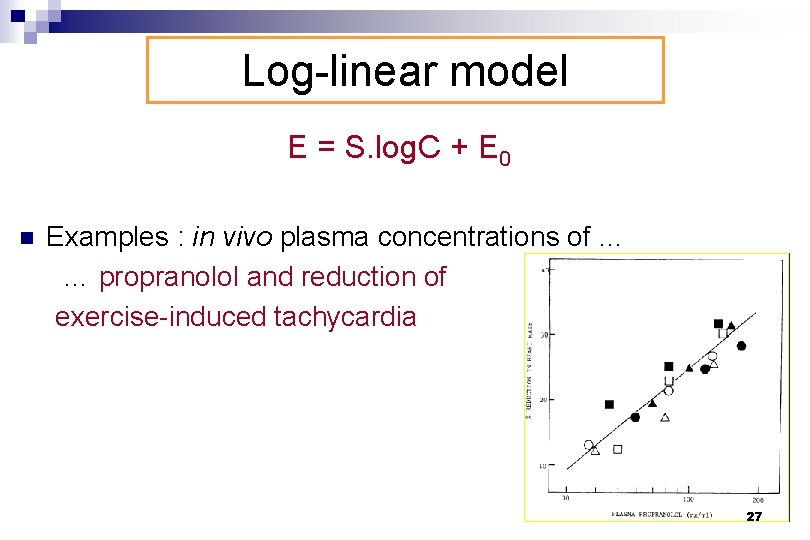
Log-linear model E = S. log. C + E 0 n Examples : in vivo plasma concentrations of … … propranolol and reduction of exercise-induced tachycardia 27

Extension of Emax model n Sigmoïd Emax model 28

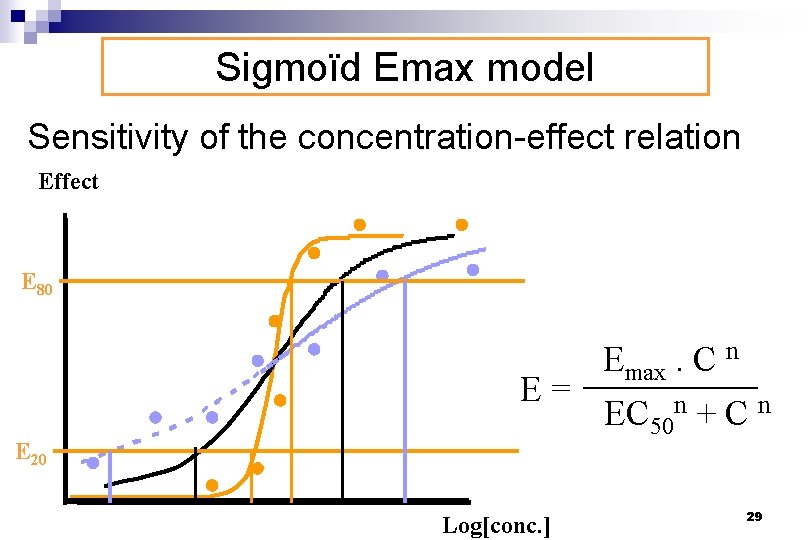
Sigmoïd Emax model Sensitivity of the concentration-effect relation Effect E 80 E 20 Emax. C n E= EC 50 n + C n Log[conc. ] 29

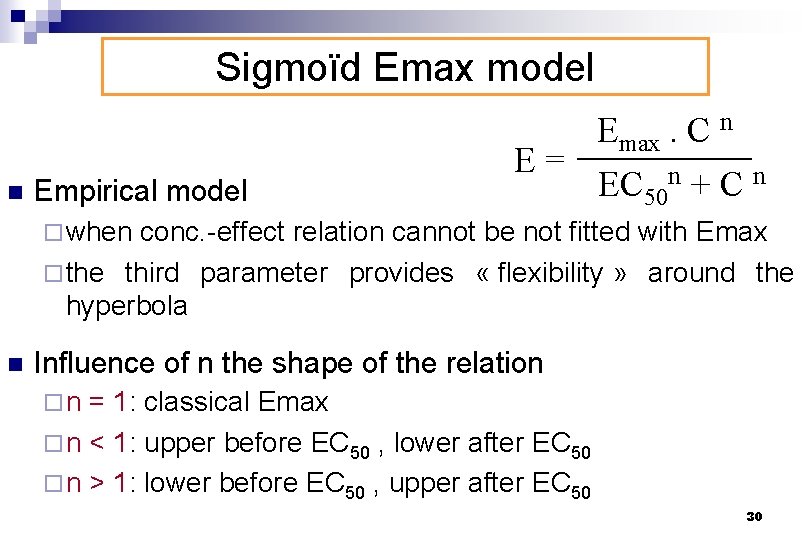
Sigmoïd Emax model n Empirical model E= Emax. C n EC 50 n + C n ¨ when conc. -effect relation cannot be not fitted with Emax ¨ the third parameter provides « flexibility » around the hyperbola n Influence of n the shape of the relation ¨ n = 1: classical Emax ¨ n < 1: upper before EC 50 , lower after EC 50 ¨ n > 1: lower before EC 50 , upper after EC 50 30

Sigmoïd Emax model n Empirical model ¨ Introduced by Archibald Hill to describe the cooperative binding of oxygen to haemoglobin : « Hill coefficient » ¨ Theoretical basis : receptor occupancy n Examples : in vivo plasma concentrations ¨ n < 1 : Conc. -effect relation very flat propranolol ¨ n > 5 : all-or-none response tocaidine /NSAID ¨ n = « SENSITIVITY » of the conc-effet. relation 31

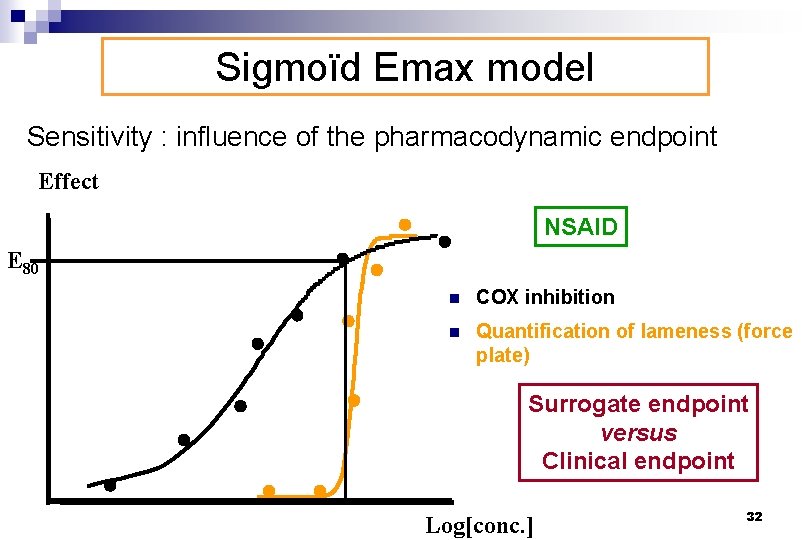
Sigmoïd Emax model Sensitivity : influence of the pharmacodynamic endpoint Effect NSAID E 80 n COX inhibition n Quantification of lameness (force plate) Surrogate endpoint versus Clinical endpoint Log[conc. ] 32

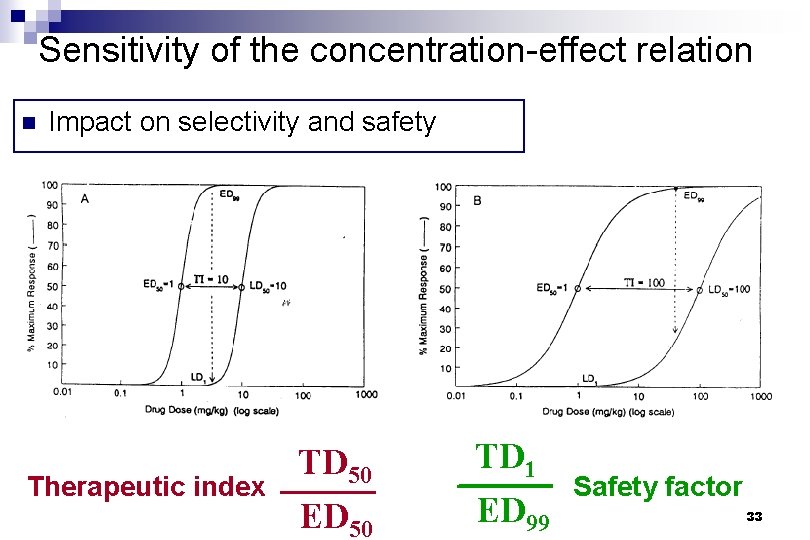
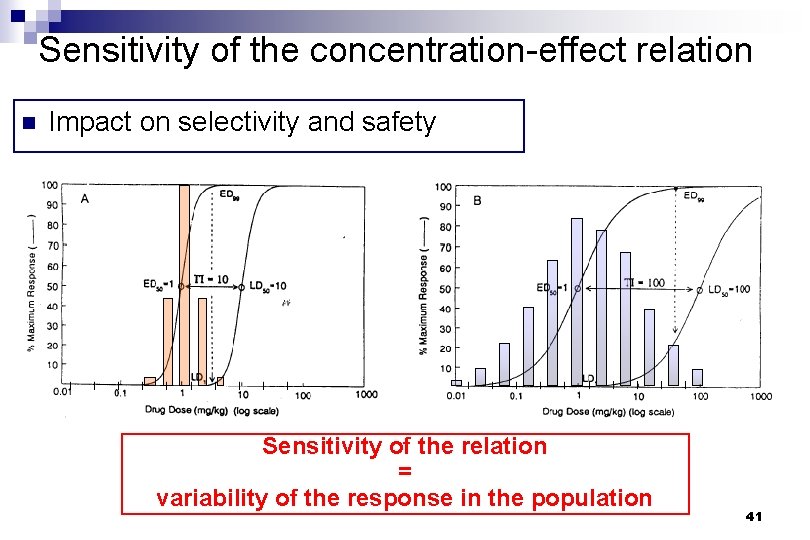
Sensitivity of the concentration-effect relation n Impact on selectivity and safety Therapeutic index TD 50 ED 50 TD 1 ED 99 Safety factor 33

Extension of Emax model Sigmoïd Emax model n Sigmoïd Emax inhibition n 34

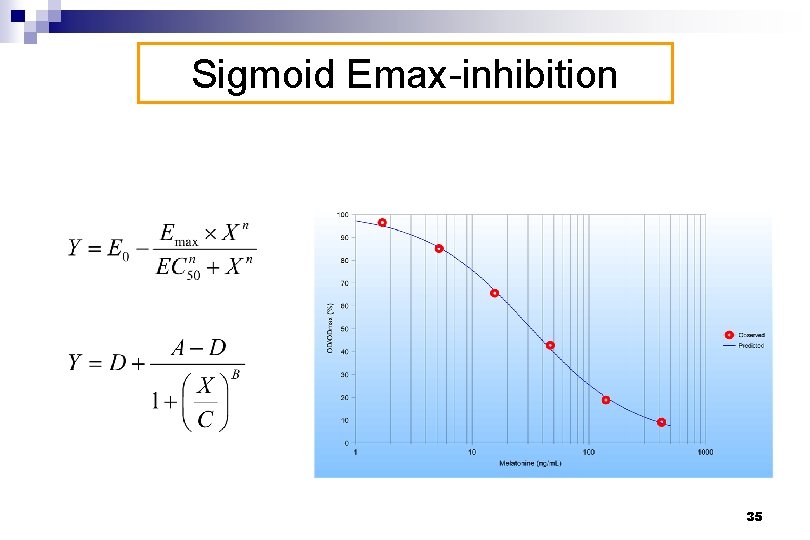
Sigmoid Emax-inhibition 35

n Relation between concentration and the intensity of an effect n Direct effects models n Indirect effects models n Relation between concentration and probability of occurrence of an effect n Fixed-effect model 36

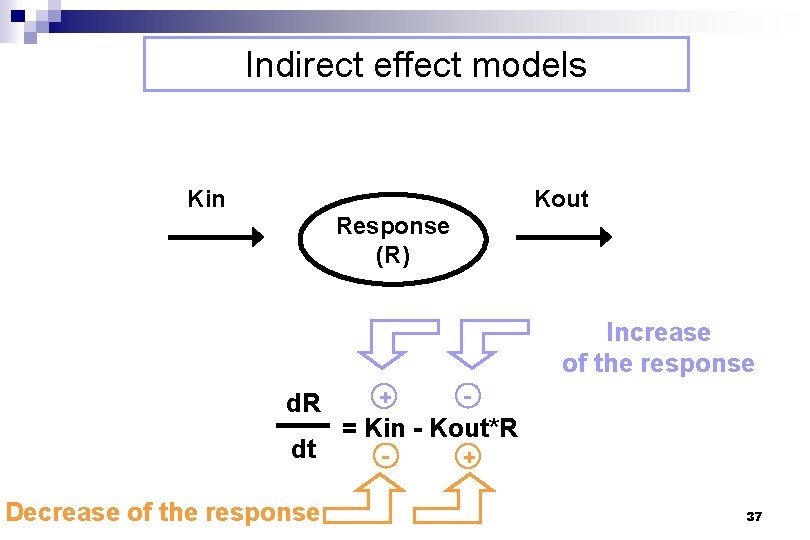
Indirect effect models Kin Kout Response (R) Increase of the response d. R dt Decrease of the response + - = Kin - Kout*R - + 37

n Relation between concentration and the intensity of an effect n Direct effects models n Indirect effects models n Relation between concentration and probability of occurrence of an effect n Fixed-effect model 38

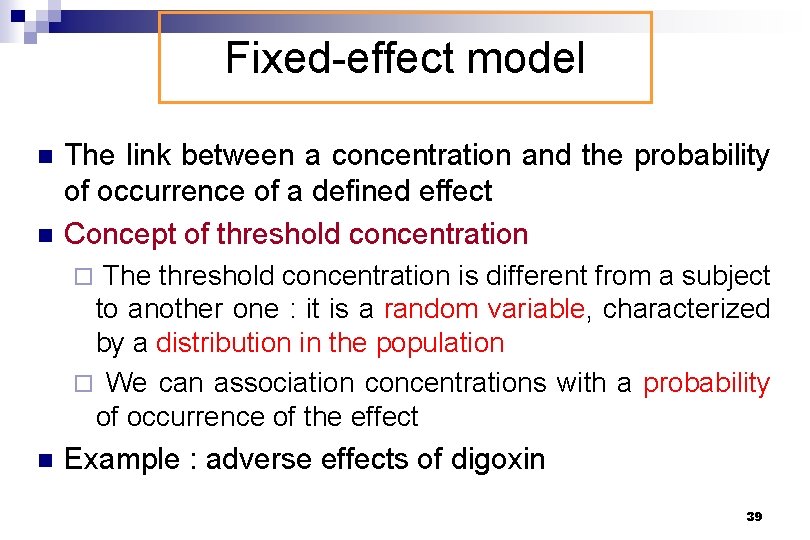
Fixed-effect model n n The link between a concentration and the probability of occurrence of a defined effect Concept of threshold concentration ¨ The threshold concentration is different from a subject to another one : it is a random variable, characterized by a distribution in the population ¨ We can association concentrations with a probability of occurrence of the effect n Example : adverse effects of digoxin 39

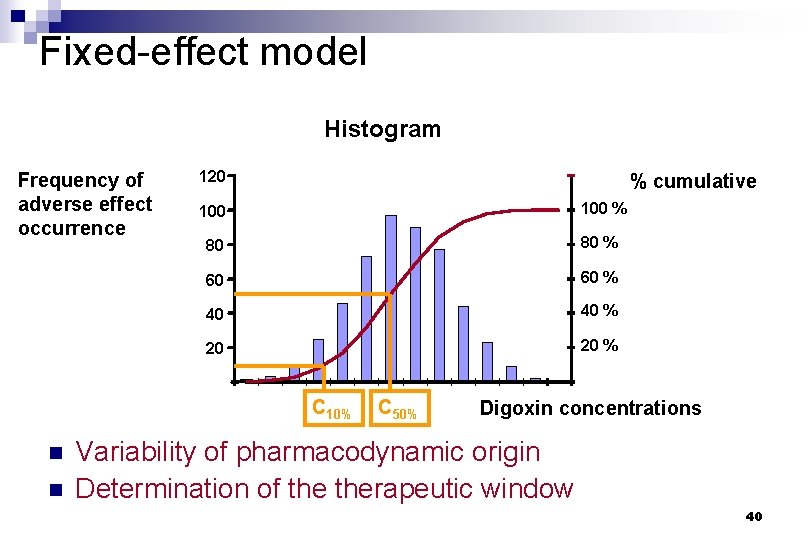
Fixed-effect model Histogram Frequency of adverse effect occurrence 120 % cumulative 100 % 80 80 % 60 60 % 40 40 % 20 20 % C 10% n n C 50% Digoxin concentrations Variability of pharmacodynamic origin Determination of therapeutic window 40

Sensitivity of the concentration-effect relation n Impact on selectivity and safety Sensitivity of the relation = variability of the response in the population 41

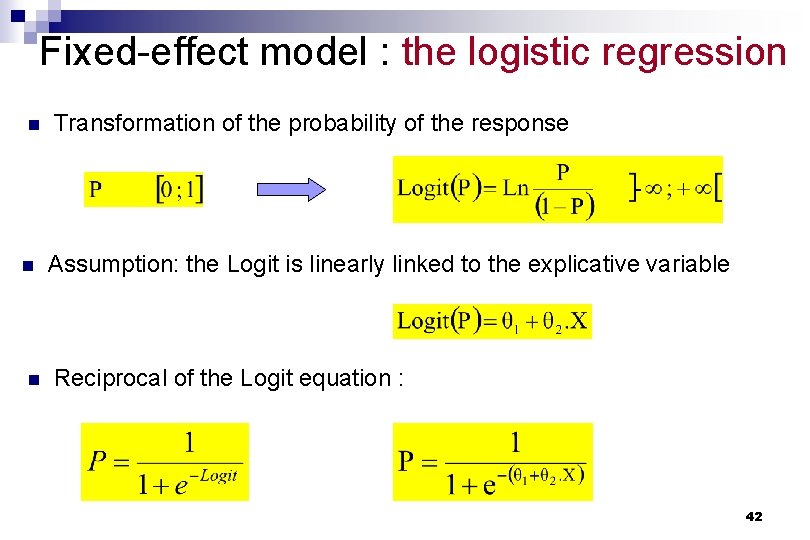
Fixed-effect model : the logistic regression n Transformation of the probability of the response Assumption: the Logit is linearly linked to the explicative variable Reciprocal of the Logit equation : 42