Pharmaceutical Development Company PDC Your trusted partner AGENDA







- Slides: 16

Pharmaceutical Development Company (PDC) “Your trusted partner”

AGENDA • Company Information • Core Value • Why should Choose PDC • Services and Goals • Therapeutic Areas • Our experience

Company Information Pharmaceutical • PDC is a leading clinicaland research organization, providing the Biotechnology full range of Phase I to IV clinical development services • Among CROs, we have the largest number of studies registered in Saudi Clinical Trials Registry (SCTR) IN SFDA CROs • We are the only CRO which cover Saudi Arabia and expanding to other area, others come to us for our unique Saudi experience Academic organizations

Company Information • Founded in 2011 • Accredited by SFDA twice • Provided wide range of Clinical Research • Consultancy Services

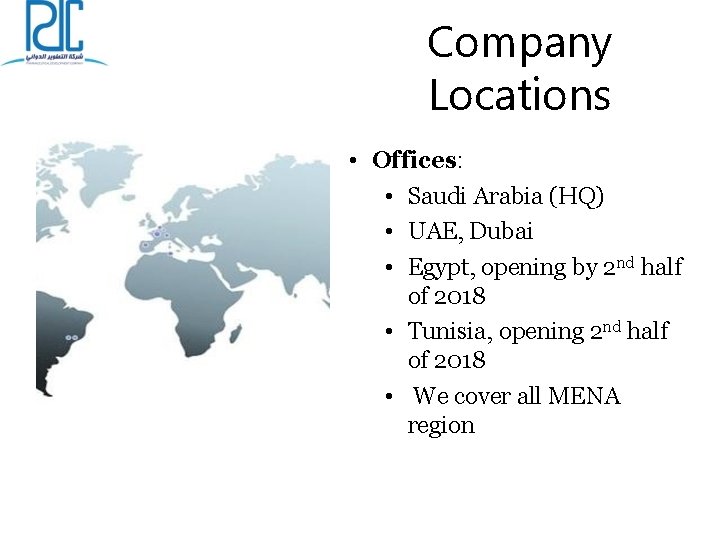
Company Locations • Offices: • Saudi Arabia (HQ) • UAE, Dubai • Egypt, opening by 2 nd half of 2018 • Tunisia, opening 2 nd half of 2018 • We cover all MENA region

Vision Our Values • To become a leader in the field, by applying and adhering to the upmost ethical and high quality standards of practice Mission • We will utilize our experience and expertise to advance and co-operate with others to the benefit of humanity Values • We strive to understand our clients' requirements to meet and exceed their expectation with high quality deliverables and service

PDC benefits from a strong local presence in Saudi Arabia and from a wide experience in Clinical Trials under international standards Strong Quality Tailored and Unique flexible experience in services KSA Our Strength

Quality is at the Heart of PDC policy Independent QA Department Own SOPs Quality Our SOPs developed & continuously upgraded Internal Audits Training programs adapted per CRA level Training programs Co-monitoring visits Periodical internal audits

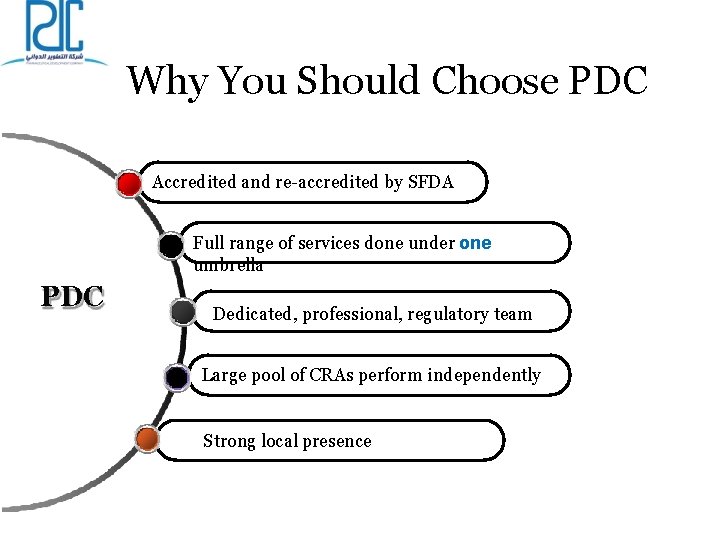
Why You Should Choose PDC Accredited and re-accredited by SFDA Full range of services done under one umbrella PDC Dedicated, professional, regulatory team Large pool of CRAs perform independently Strong local presence

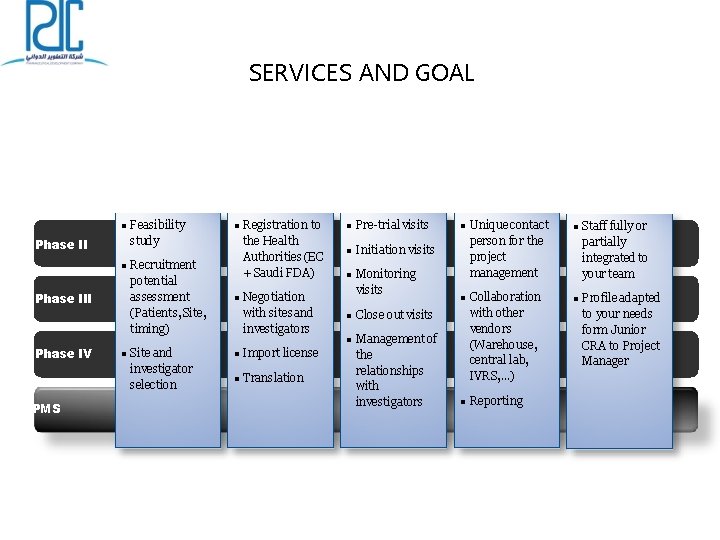
SERVICES AND GOAL Feasibility Phase III Feasibility study Recruitment potential assessment (Patients, Site, timing) Regulatory Registration to the Health Authorities (EC + Saudi FDA) Negotiation with sites and investigators Monitoring Pre-trial visits Initiation visits Phase IV PMS Site and investigator selection Import license Translation Monitoring visits Project Management Close out visits Management of the relationships with investigators Unique contact person for the project management Collaboration with other vendors (Warehouse, central lab, IVRS, …) Reporting CRA outsourcing Staff fully or partially integrated to your team Profile adapted to your needs form Junior CRA to Project Manager

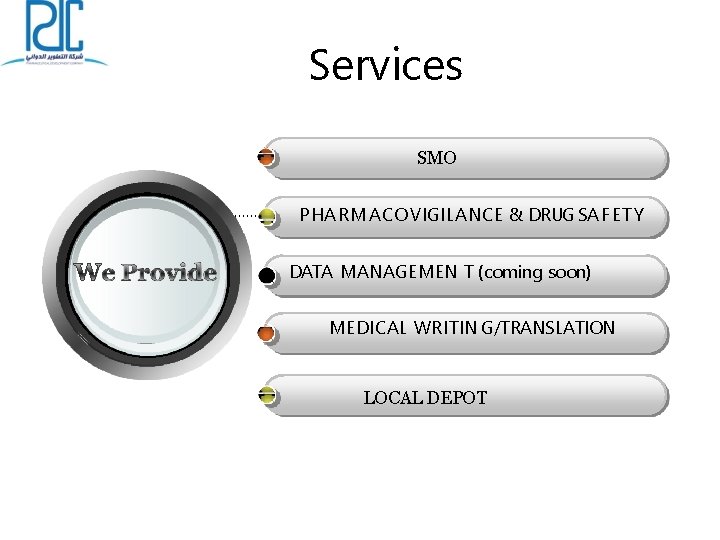
Services SMO P HA R M A CO VIGILANCE & DRUG SA F ET Y DATA MANAGEMEN T (coming soon) MEDICAL WRITIN G/TRANSLATION LOCAL DEPOT

CRA outsourcing Thanks to our expertise in Clinical Trials, we can select the best candidates. Why is PDC the best partner for your CRA outsourcing needs? 1 Widest candidate data base: developed since 2011 + unique local presence allowing us to screen all needs to our clients 2 Best recruitment process: our highly experienced team can select the best candidates 3 Strong experience in insourcing / outsourcing: in Saudi Arabia. We also do SMO module 4 Excellent access to Saudi Market: accredited by SFDA, Saudi Country Manager, Saudi CRAs, Saudi General Manager…

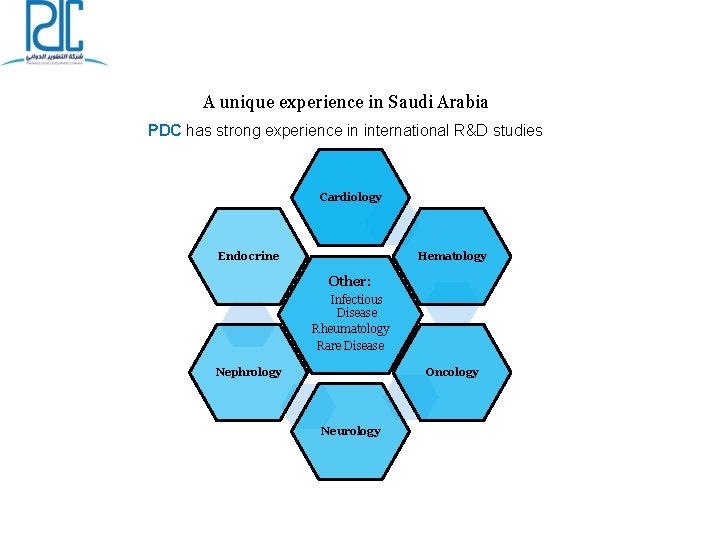
A unique experience in Saudi Arabia PDC has strong experience in international R&D studies Cardiology Endocrine Hematology Other: Infectious Disease Rheumatology Rare Disease Oncology Nephrology Neurology

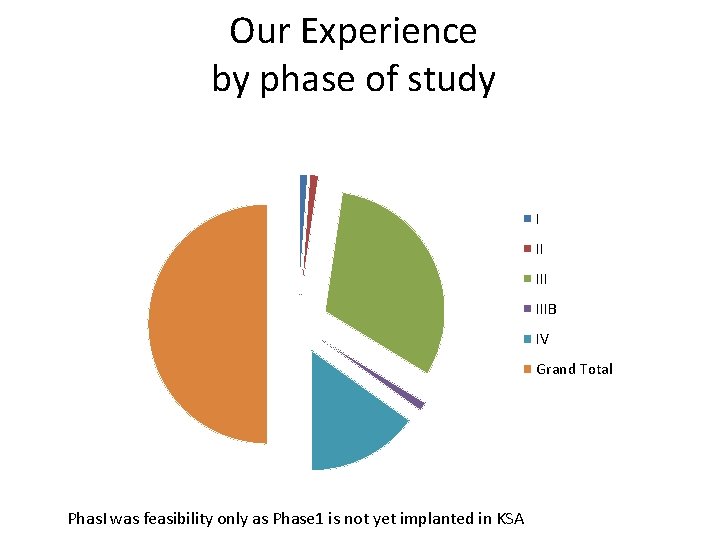
Our Experience by phase of study I II IIIB IV Grand Total Phas. I was feasibility only as Phase 1 is not yet implanted in KSA

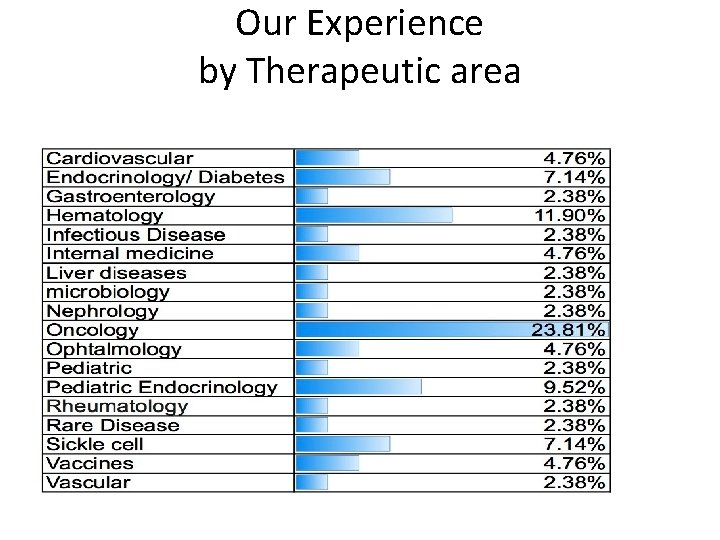
Our Experience by Therapeutic area

Contact Information http: //www. pdcme. net/ info@pdcme. net +966114190064