PHARMACEUTICAL CHEMISTRY LECTURE 3 Methods of identification of


















- Slides: 37

PHARMACEUTICAL CHEMISTRY LECTURE 3 « Methods of identification of medicinal products. »

Identification of Medicinal Products (IDMP) • IDMP is a suite of five standards developed within the International Organization for Standardization (ISO). These standards provide an internationally-accepted framework to uniquely identify and describe medicinal products with consistent documentation, coding and exchange of product information between global regulators, manufacturers, suppliers and distributors. The IDMP suite of standards are a result of a need to standardize the definition of medicinal product and substance information to facilitate the unique identification and exchange of such information in the context of pharmacovigilance. As FDA focuses on the challenges of the global supply chain and foreign sourcing of medicinal products, FDA continues to participate in the development of and to promote the adoption of international harmonized IDMP to ensure the safety of medications throughout the world.

Medicinal Product Identification (MPID) • ISO 11615 External Link Disclaimer: Data elements and structures for unique identification and exchange of regulated medicinal product information. MPID describes the detailed data elements and their structural relationships required for the unique identification of regulated medicinal products. Data elements that identify and characterize a medicinal product include the product name (authorized by regulatory agency), clinical particulars (e. g. indications, contraindications), pharmaceutical product (substance, dosage form, route of administration), medicinal product packaging, marketing authorization (e. g. , authorization number, application information), manufacturer/establishment, etc.

Pharmaceutical Product Identifier (Ph. PID) • ISO 11616 External Link Disclaimer: Data elements and structures for unique identification and exchange of regulated pharmaceutical product information. Ph. PID uniquely associates medical products with the same or similar pharmaceutical composition based on the following data elements: substance(s), strength(s) (units of measurement/presentation), reference strength(s), and dosage form.

Methods for the analysis of medicinal substances. The purpose of the study of medicinal substances is to establish the suitability of a medicinal product for medical use, i. e. compliance with the regulatory document for this drug. Pharmaceutical analysis is the science of chemical characterization and measurement of biologically active substances at all stages of production: from raw material control to assessing the quality of the resulting medicinal substance, studying its stability, establishing shelf life and standardizing the finished dosage form. The peculiarities of pharmaceutical analysis are its versatility and variety of substances or their mixtures, including individual chemicals, complex mixtures of biological substances (proteins, carbohydrates, oligopeptides, etc. ).

• Methods of analysis need constant improvement and, if chemical methods, including qualitative reactions, prevailed in the UP pharmacopoeia, at the present stage, physicochemical and physical methods of analysis are mainly used.

Pharmaceutical analysis, depending on the tasks, includes various aspects of drug quality control: • 1. Pharmacopoeial analysis; • 2. Stepwise control of the production of medicines; • 3. Analysis of individually manufactured medicines. • The main and most essential is the pharmacopoeial analysis, i. e. analysis of medicinal products for compliance with the standard monograph or other regulatory documents and, thus, confirmation of its suitability. Hence the requirements for high specificity, selectivity, accuracy and reliability of the analysis.

• A conclusion about the quality of a medicinal product can only be made on the basis of sample analysis (statistically reliable sample). The sampling procedure is specified either in a private article or in a general article of the GF X 1 ed. (issue 2) p. 15. To test medicinal products for compliance with the requirements of normative and technical documentation, a multistage sampling (sampling) is carried out. In multi-stage sampling, a sample (sampling) is formed in stages and the products in each stage are selected randomly in proportional quantities from the units selected in the previous stage. The number of steps is determined by the type of packaging. • Stage 1: selection of packaging units (boxes, etc. ); • Stage 2: selection of packaging units in packaging containers (boxes, vials, cans, etc. ); • Stage 3: selection of products in primary packaging (ampoules, vials, contour packages, etc. ).

• To calculate the selection of the number of products at each stage, use the formula: 0, 4√n where n - is the number of packaging units of a given step. The specific sampling procedure is described in detail in the GF X 1 edition, issue 2. In this case, the analysis is considered reliable if the reproducibility of at least four samples.

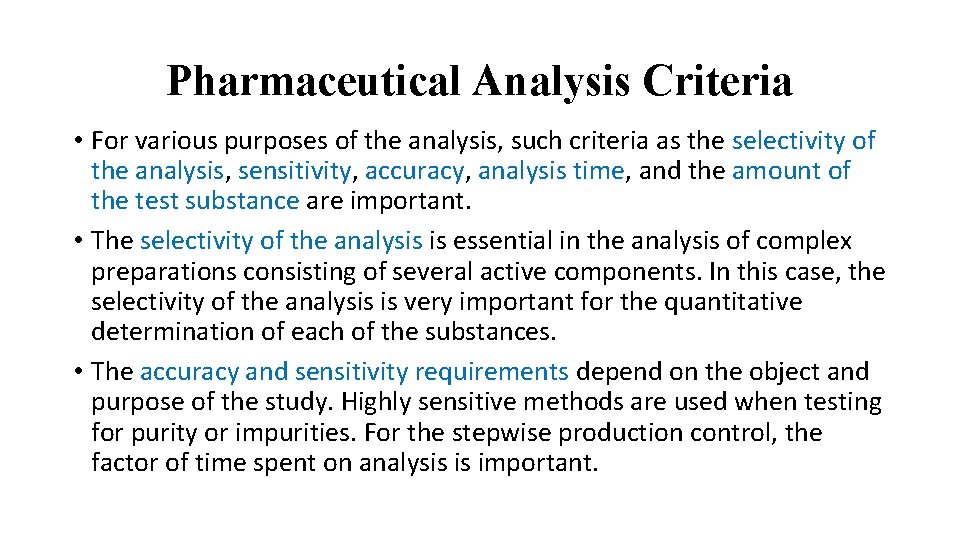
Pharmaceutical Analysis Criteria • For various purposes of the analysis, such criteria as the selectivity of the analysis, sensitivity, accuracy, analysis time, and the amount of the test substance are important. • The selectivity of the analysis is essential in the analysis of complex preparations consisting of several active components. In this case, the selectivity of the analysis is very important for the quantitative determination of each of the substances. • The accuracy and sensitivity requirements depend on the object and purpose of the study. Highly sensitive methods are used when testing for purity or impurities. For the stepwise production control, the factor of time spent on analysis is important.

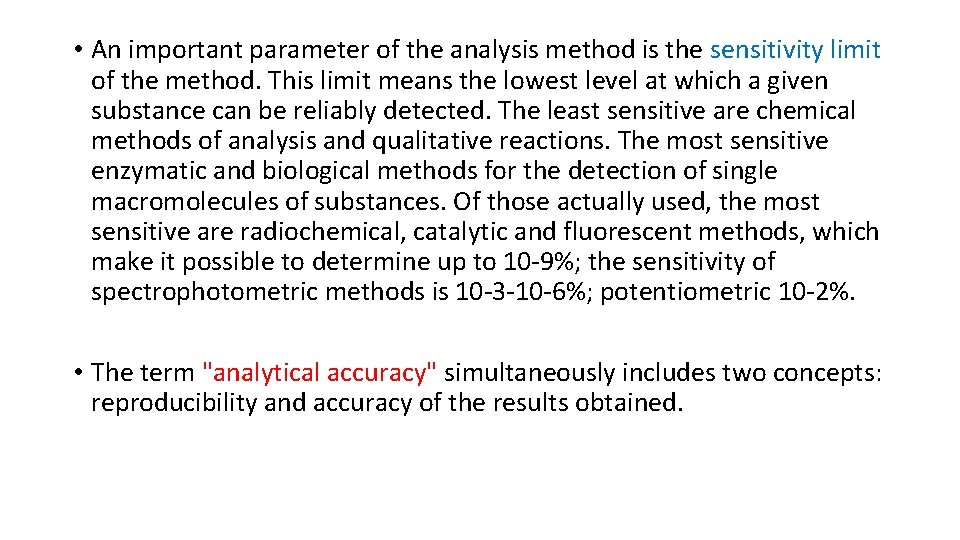
• An important parameter of the analysis method is the sensitivity limit of the method. This limit means the lowest level at which a given substance can be reliably detected. The least sensitive are chemical methods of analysis and qualitative reactions. The most sensitive enzymatic and biological methods for the detection of single macromolecules of substances. Of those actually used, the most sensitive are radiochemical, catalytic and fluorescent methods, which make it possible to determine up to 10 -9%; the sensitivity of spectrophotometric methods is 10 -3 -10 -6%; potentiometric 10 -2%. • The term "analytical accuracy" simultaneously includes two concepts: reproducibility and accuracy of the results obtained.

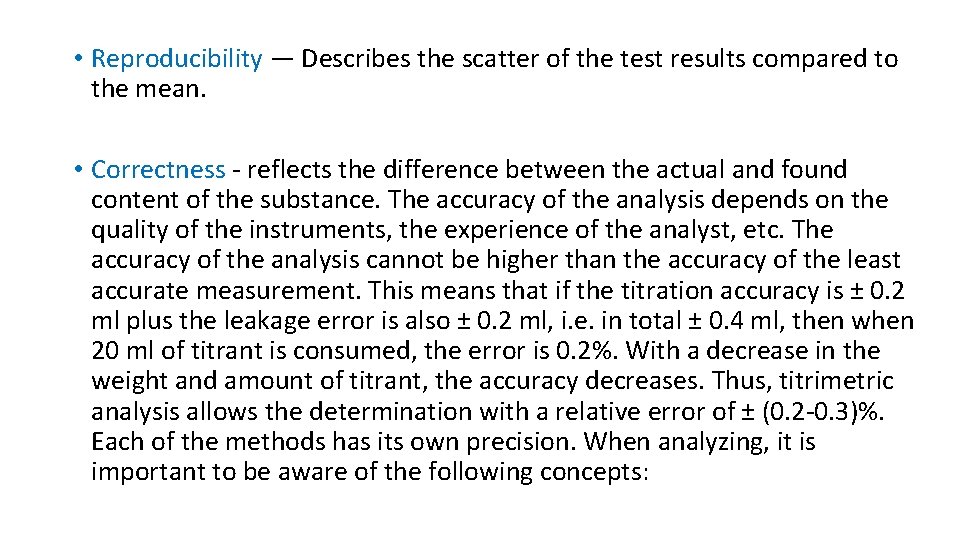
• Reproducibility — Describes the scatter of the test results compared to the mean. • Correctness - reflects the difference between the actual and found content of the substance. The accuracy of the analysis depends on the quality of the instruments, the experience of the analyst, etc. The accuracy of the analysis cannot be higher than the accuracy of the least accurate measurement. This means that if the titration accuracy is ± 0. 2 ml plus the leakage error is also ± 0. 2 ml, i. e. in total ± 0. 4 ml, then when 20 ml of titrant is consumed, the error is 0. 2%. With a decrease in the weight and amount of titrant, the accuracy decreases. Thus, titrimetric analysis allows the determination with a relative error of ± (0. 2 -0. 3)%. Each of the methods has its own precision. When analyzing, it is important to be aware of the following concepts:

• Gross errors are an observer's miscalculation or a violation of the analysis method. Such results are discarded as invalid. • Systematic errors - reflect the correctness of the analysis results. They distort the measurement results, as a rule, in one direction by some constant value. Systematic errors can be partially eliminated by introducing corrections, calibrating the instrument, etc. • Random errors - reflect the reproducibility of the analysis results. They are called by uncontrolled variables. The arithmetic mean of random errors tends to zero. Therefore, for calculations it is necessary to use not the results of single measurements, but the average of several parallel determinations. • Absolute error is the difference between the result and the true value. This error is expressed in the same units as the value to be determined.

• The relative error of determination is equal to the ratio of the absolute error to the true value of the determined value. It is usually expressed as a percentage or fraction. The values of relative errors depend on what method the analysis is performed and what the analyte is - an individual substance and a mixture of many components. The relative error in studies of individual substances by the spectrophotometric method is 2 -3%, by IR spectrophotometry - 5 -12%; liquid chromatography 3 -4%; potentiometry 0. 3 -1%. Combined methods tend to reduce the accuracy of the analysis. Biological methods are the least accurate - their relative error reaches 50%.

Methods for the identification of medicinal substances. • The most important indicator in the testing of medicinal substances is their identification or, as is customary in monographs, the authenticity. Numerous methods are used to determine the authenticity of medicinal substances. All basic and general ones are described in the GF X 1 edition, issue 1. Historically, the main focus has been on chemical, incl. qualitative color reactions, characterizing the presence of certain ions or functional groups in organic compounds, at the same time, physical methods were widely used. In modern pharmacopoeias, the emphasis is on physicochemical methods.

Let us dwell on the basic physical methods. • Melting point is a fairly stable constant characterizing a substance, its purity and authenticity. This indicator is widely used to standardize drug substances. Methods for determining the melting point are described in detail in GF X 1; yourself were able to test it in laboratory studies. A pure substance has a constant melting point; however, when impurities are added to it, the melting point usually decreases quite significantly. This effect is called a mixing test and it is the mixing test that allows you to establish the authenticity of the drug in the presence of a standard sample or a known sample. There are, however, exceptions, so racemic sulfocamphoric acid melts at a higher temperature, and various crystalline forms of indomethacin differ in melting point. Those. this method is one of the indicators that characterize both the purity of the product and its authenticity. • For some drugs, such an indicator as the solidification temperature is used. Another indicator that characterizes a substance is the boiling point or temperature limits of distillation. This indicator characterizes liquid substances, for example, ethyl alcohol. The boiling point is less characteristic, it strongly depends on the atmospheric pressure, the possibility of the formation of mixtures or azeotropes, and is rarely used.

• Among other physical methods, the determination of density and viscosity should be noted. Standard methods of analysis are described in GF X 1. The method characterizing the authenticity of the drug is also the determination of its solubility in various solvents. According to GF X 1 ed. This method is characterized as a property that can serve as an indicative characteristic of the test drug. Along with the melting point, the solubility of a substance is one of the parameters by which the authenticity and purity of almost all medicinal substances is established. In the pharmacopoeia, an approximate gradation of substances according to solubility is established from very easily soluble to practically insoluble. In this case, a substance is considered to be dissolved, in a solution of which particles of a substance are not observed in transmitted light.

Physicochemical methods for determining the authenticity. • The most informative in terms of determining the authenticity of substances are physicochemical methods based on the properties of molecules of substances to interact with any physical factors. Physicochemical methods include: 1. Spectral methods • UV spectroscopy • Visible light spectroscopy • IR spectroscopy • Fluorescence spectroscopy • Atomic absorption spectroscopy • X-ray analysis methods • Nuclear magnetic resonance • X-ray structural analysis

2. Sorption methods of analysis • Thin layer chromatography • Gas Liquid Chromatography • High performance liquid chromatography • Eletrophoresis • Iontophoresis • Gel chromatography • 3. Mass analysis methods • Mass spectrometry • Chromatomass spectrometry 4. Electrochemical analysis methods • Polarography • Electronic paramagnetic resonance 5. Use of reference materials

• Let us briefly consider the methods of analysis applicable in pharmacy. All these methods of analysis will be read to you in detail at the end of December by Professor Myagkikh V. I. To determine the authenticity of medicinal substances, some spectral methods are used. The most reliable is the use of the low-frequency region of IR spectroscopy, where the absorption bands most reliably reflect the given substance. I also call this area the fingerprint area. As a rule, to confirm the authenticity, a comparison of the IR spectra taken under standard conditions of the standard sample and the test sample is used. The coincidence of all absorption bands confirms the authenticity of the drug. The use of UV and visible spectroscopy is less reliable because the nature of the spectrum is not individual and reflects only a certain chromophore in the structure of an organic compound. Atomic absorption spectroscopy and X-ray spectroscopy are used to analyze inorganic compounds, to identify chemical elements. Nuclear magnetic resonance makes it possible to establish the structure of organic compounds and is a reliable method of authenticity confirmation, however, due to the complexity of the instruments and high cost, it is used very rarely and, as a rule, only for research purposes. Fluorescence spectroscopy is only applicable to a certain class of substances that fluoresce when exposed to UV radiation. In this case, the fluorescence spectrum and fluorescence excitation spectrum are quite individual, but strongly depend on the medium in which the given substance is dissolved. This method is more often used for quantitative determination, especially of small quantities, as it is one of the most sensitive.

• Sorption methods of analysis have found very wide application in pharmaceutical analysis. They are used to determine authenticity, impurities, and quantification. You will be given a lecture by Professor V. I. Myagkikh, a regional representative of Shimadzu, one of the main manufacturers of chromatographic equipment, in detail about these methods and the equipment used. These methods are based on the principle of sorption-desorption of substances on specific carriers in a carrier stream. Depending on the carrier and sorbent, they are subdivided into thin layer chromatography, liquid column chromatography (analytical and preparative, including HPLC), gas-liquid chromatography, gel filtration, iontophoresis. The last two methods are used to analyze complex protein objects. A significant drawback of the methods is their relativity, i. e. chromatography can characterize a substance and its amount only when compared with a standard substance. However, it should be noted as a significant advantage - high reliability of the method and accuracy, since in chromatography, any mixture must be separated into individual substances and the result of the analysis is precisely the individual substance.

• Mass spectrometric and electrochemical methods are rarely used for authentication. • A special place is occupied by methods for determining the authenticity in comparison with a standard sample. This method is widely used in foreign pharmacopoeias to determine the authenticity of complex macromolecules, complex antibiotics, some vitamins, and other substances containing especially chiral carbon atoms, since it is difficult or completely impossible to determine the authenticity of an optically active substance by other methods. The standard sample must be developed and produced on the basis of a developed and approved pharmacopoeial monograph. In Russia, only a few standard samples exist and are used, and most often so-called RSOs are used for analysis - working standard samples prepared immediately before the experiment from known substances or corresponding substances.

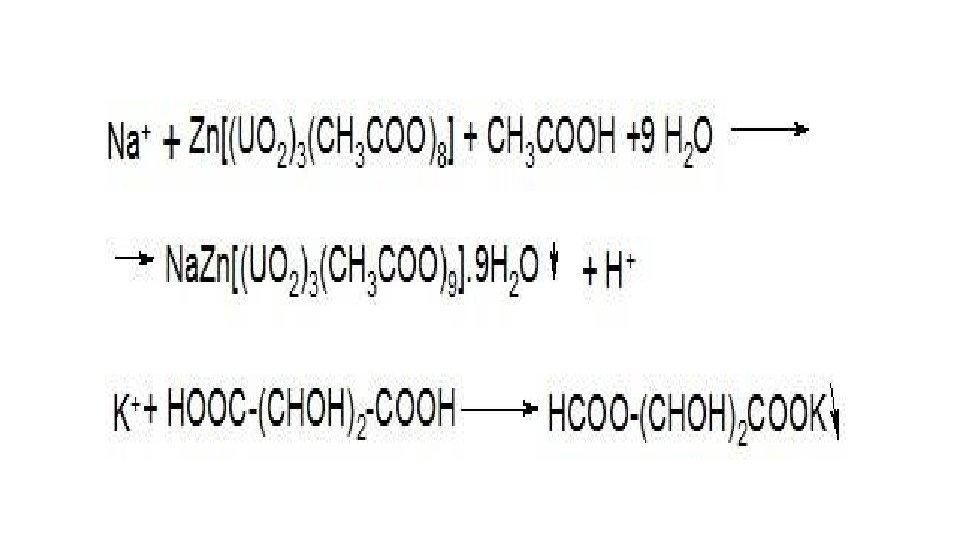
Chemical methods of authentication. • Reactions of precipitation of anions and cations. Typical examples are sodium and potassium ion precipitation reactions with (zinc-curanyl acetate and tartaric acid), respectively:


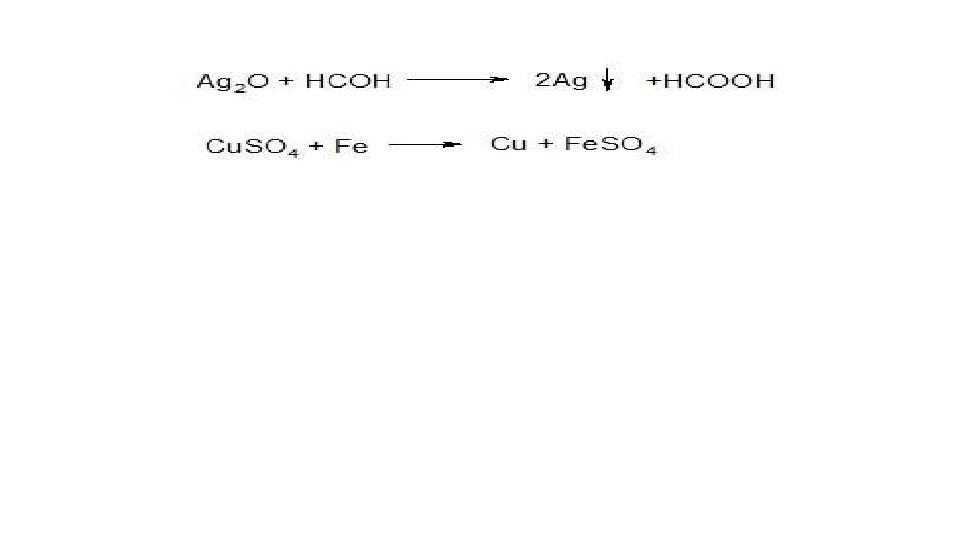
• A great variety of such reactions are used and they will be discussed in detail in a special section of pharmaceutical chemistry regarding inorganic substances. Redox reactions are used to reduce metals from oxides. For example, silver from its formalin oxide (silver mirror reaction):


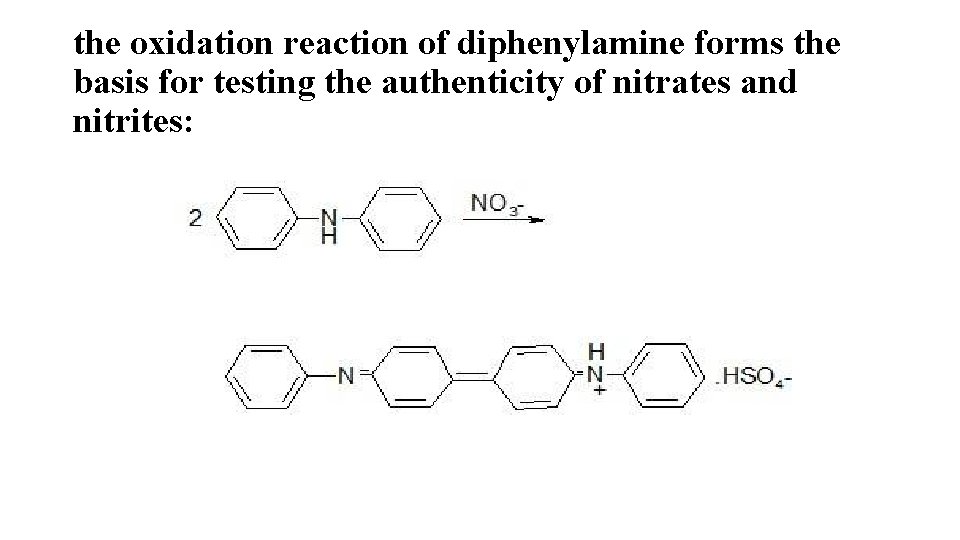
the oxidation reaction of diphenylamine forms the basis for testing the authenticity of nitrates and nitrites:

Reactions of neutralization and decomposition of anions. • Carbonates and bicarbonates under the action of mineral acids form carbonic acid, which decomposes to carbon dioxide.

Identification of organoelemental medicinal substances. Qualitative elemental analysis is used to identify compounds containing arsenic, sulfur, bismuth, mercury, phosphorus, and halogens in an organic molecule. Since the atoms of these elements are not ionized, preliminary mineralization is used for their identification, either by pyrolysis, or again by pyrolysis with sulfuric acid. Sulfur is determined by hydrogen sulfide by reaction with potassium nitroprusside or lead salts. Iodine is also determined by pyrolysis by the release of elemental iodine. Of all these reactions, the identification of arsenic is of interest, not so much as a drug - they are practically not used, but as a method for controlling impurities, but more on that later. • Testing the authenticity of organic medicinal substances. The chemical reactions used to test the authenticity of organic medicinal substances can be divided into three main groups: • 1. General chemical reactions of organic compounds; • 2. Reactions of formation of salts and complex compounds; • 3. Reactions used to identify organic bases and their salts. All these reactions are ultimately based on the principles of functional analysis, i. e. the reactive center of the molecule, which, by reacting, gives an appropriate response. Most often, this is a change in any properties of the substance: color, solubility, state of aggregation, etc.

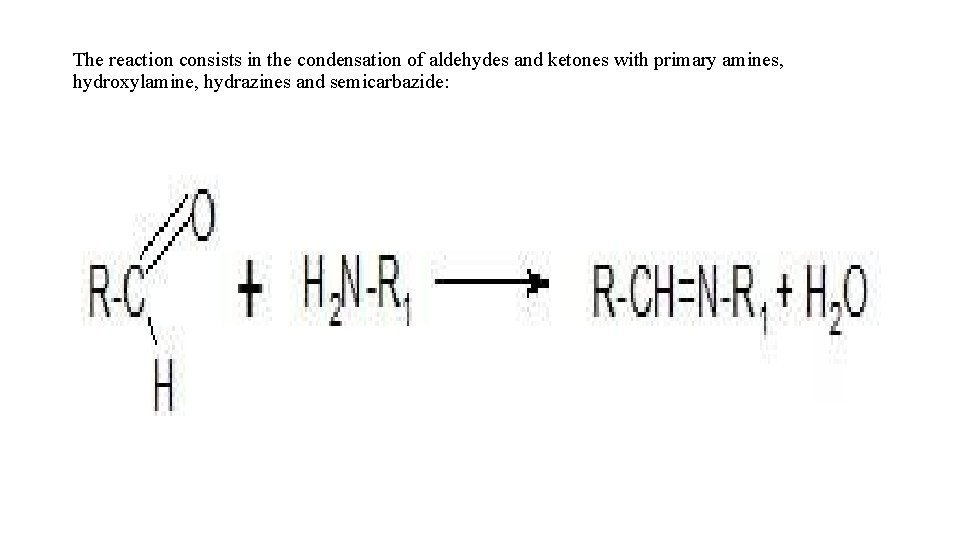
• Let's consider some examples of using chemical reactions to identify medicinal substances. • 1. Reactions of nitration and nitrosation. They are used quite rarely, for example, to identify phenobarbital, phenacetin, dicain, although these drugs are almost never used in medical practice. • 2. Reactions of diazotization and azo coupling. These reactions are used to open up primary amines. The diazotized amine combines with beta-naphthol to give a characteristic red or orange color. • 3. Reactions of halogenation. They are used to open aliphatic double bonds - when bromine water is added, bromine is added to the double bond and the solution becomes discolored. A characteristic reaction of aniline and phenol is that when they are treated with bromine water, a tribromo derivative is formed, which precipitates. • 4. Reactions of condensation of carbonyl compounds. The reaction consists in the condensation of aldehydes and ketones with primary amines, hydroxylamine, hydrazines and semicarbazide:

The reaction consists in the condensation of aldehydes and ketones with primary amines, hydroxylamine, hydrazines and semicarbazide:

• The resulting azomethines (or Schiff bases) have a characteristic yellow color. The reaction is used to identify, for example sulfonylamides. 4 -dimethylaminobenzaldehyde is used as the aldehyde.

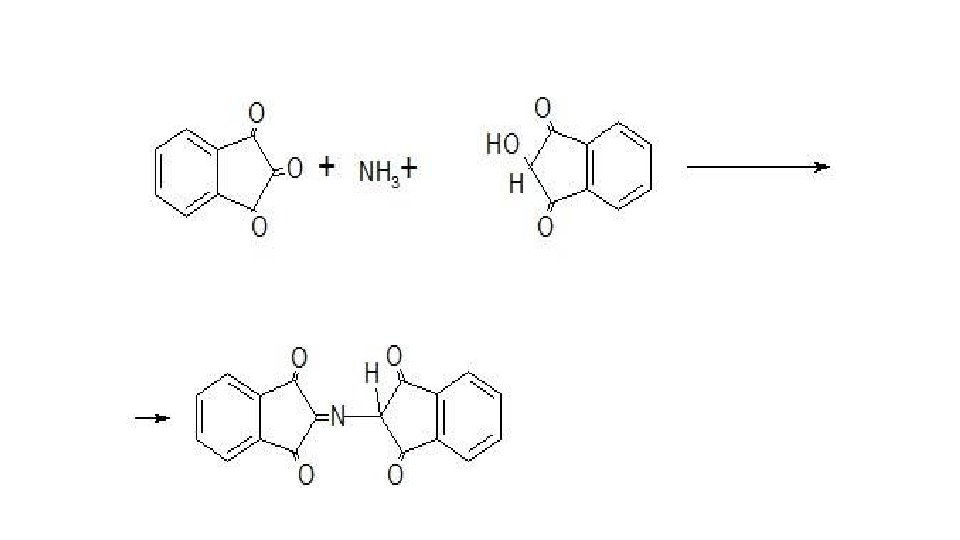
• 5. Reactions of oxidative condensation. The process of oxidative degradation and formation of the azomethine dye underlies the ninhydrin reaction. This reaction is widely used for the discovery and photocolorimetric determination of α- and β-amino acids, in the presence of which an intense dark blue color appears. It is due to the formation of a substituted salt of diketohydrindylidene diketohydramine, a condensation product of excess ninhydrin and reduced ninhydrin with ammonia released during the oxidation of the tested amino acid:


• For the discovery of phenols, the reaction of formation of triarylmethane dyes is used. So phenols interact with formaldehyde to form dyes. Analogous reactions include the interaction of resorcinol with phthalic anhydride, leading to the formation of a fluorescent dye - fluorescein. • Many other reactions are also used. • Reactions with the formation of salts and complexes are of particular interest. Inorganic salts of iron (III), copper (II), silver, cobalt, mercury (II) and others for testing the authenticity of organic compounds: carboxylic acids, including amino acids, barbituric acid derivatives, phenols, sulfonylamides, some alkaloids. The formation of salts and complex compounds occurs according to the general scheme:

R-COOH + MX = R-COOM + HX Complexation of amines proceeds in a similar way: R-NH 2 + X = R-NH 2·X Iron (III) chloride solution is one of the most common reagents in pharmaceutical analysis. Interaction with phenols, it forms a colored solution of phenoxides, they are colored blue or purple. This reaction is used to open phenol or resorcinol. However, meta-substituted phenols do not form colored compounds (thymol). Copper salts form complex compounds with sulfonylamides, cobalt salts with barbiturates. Many of these reactions are also used for quantitative determination.

Identification of organic bases and their salts. This group of methods is most often used in finished forms, especially when researching solutions. So salts of organic amines with the addition of alkalis form a base precipitate (for example, a solution of papaverine hydrochloride) and vice versa, salts of organic acids with the addition of a mineral acid give a precipitate of an organic compound (for example, sodium salicylate). For the identification of organic bases and their salts, so-called precipitation reagents are widely used. More than 200 precipitation reagents are known that form water-insoluble simple or complex salts with organic compounds. The most common solutions are given in the second volume of the GF 11 edition. Examples include: • Scheibler's reagent - phosphotungstic acid; • Picric acid • Styphnic acid • Picramic acid All of these reagents are used to precipitate organic bases (eg nitroxoline).