PHAR 827 IMMUNOLOGY LEO R FITZPATRICK Ph D
![Acanthosis (epidermal hyperplasia [thickening of the skin, particularly in the stratum spinosum], Parakeratosis (keratinization Acanthosis (epidermal hyperplasia [thickening of the skin, particularly in the stratum spinosum], Parakeratosis (keratinization](https://slidetodoc.com/presentation_image/5ee78cc3b862d5a63f763d4b1be41e7c/image-4.jpg)

- Slides: 22

PHAR 827: IMMUNOLOGY LEO R. FITZPATRICK Ph. D. ASSOCIATE PROFESSOR Class # 17 – Th 1/Th 17 mediated diseases: Psoriasis

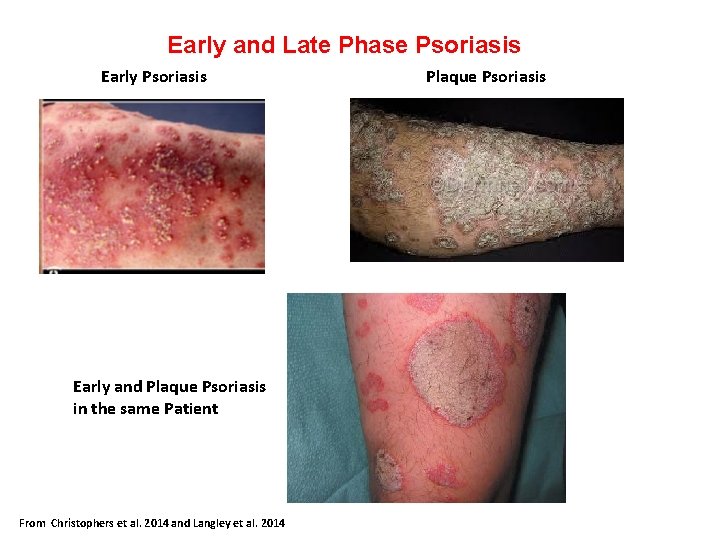
Early and Late Phase Psoriasis Early Psoriasis Plaque Psoriasis Early and Plaque Psoriasis in the same Patient From Christophers et al. 2014 and Langley et al. 2014

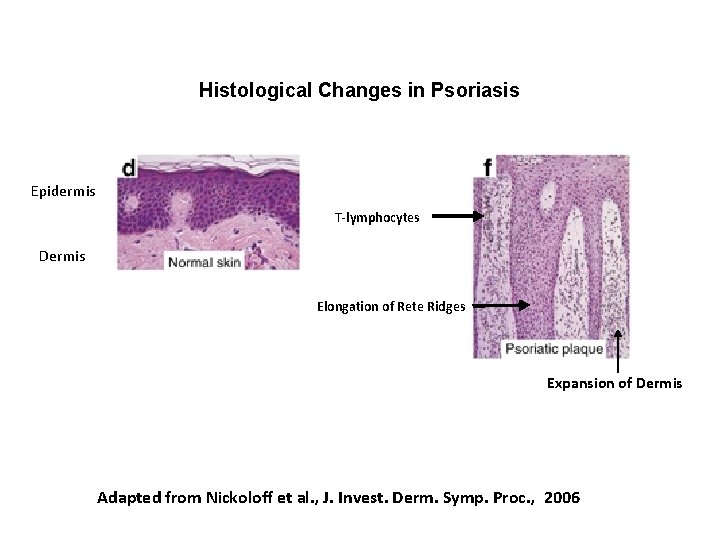
Histological Changes in Psoriasis Epidermis T-lymphocytes Dermis Elongation of Rete Ridges Expansion of Dermis Adapted from Nickoloff et al. , J. Invest. Derm. Symp. Proc. , 2006
![Acanthosis epidermal hyperplasia thickening of the skin particularly in the stratum spinosum Parakeratosis keratinization Acanthosis (epidermal hyperplasia [thickening of the skin, particularly in the stratum spinosum], Parakeratosis (keratinization](https://slidetodoc.com/presentation_image/5ee78cc3b862d5a63f763d4b1be41e7c/image-4.jpg)
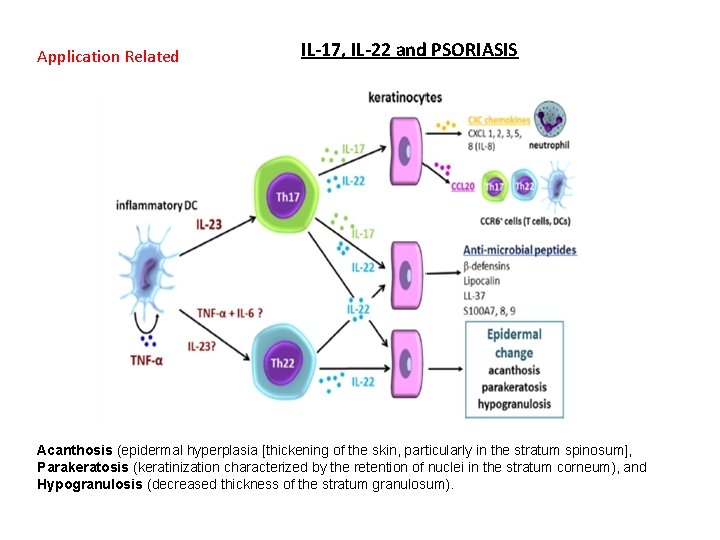
Acanthosis (epidermal hyperplasia [thickening of the skin, particularly in the stratum spinosum], Parakeratosis (keratinization characterized by the retention of nuclei in the stratum corneum), and Hypogranulosis (decreased thickness of the stratum granulosum).

Psoriasis: Background Information Role of the Inflammasome in Psoriasis Th 17 cell plasticity ? ? From E. Christophers et al. , Br. J. Dermatology, 2014

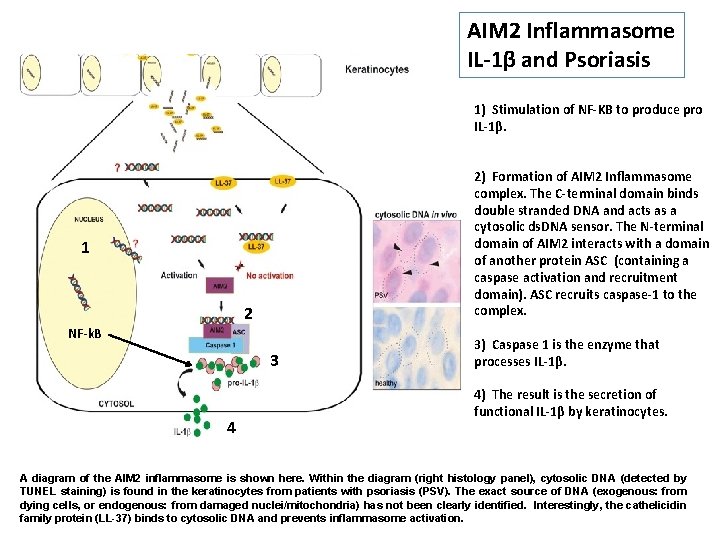
AIM 2 Inflammasome IL-1β and Psoriasis 1) Stimulation of NF-KB to produce pro IL-1β. 2) Formation of AIM 2 Inflammasome complex. The C-terminal domain binds double stranded DNA and acts as a cytosolic ds. DNA sensor. The N-terminal domain of AIM 2 interacts with a domain of another protein ASC (containing a caspase activation and recruitment domain). ASC recruits caspase-1 to the complex. 1 NF-k. B 2 NFκB 3 4 3) Caspase 1 is the enzyme that processes IL-1β. 4) The result is the secretion of functional IL-1β by keratinocytes. A diagram of the AIM 2 inflammasome is shown here. Within the diagram (right histology panel), cytosolic DNA (detected by TUNEL staining) is found in the keratinocytes from patients with psoriasis (PSV). The exact source of DNA (exogenous: from dying cells, or endogenous: from damaged nuclei/mitochondria) has not been clearly identified. Interestingly, the cathelicidin family protein (LL-37) binds to cytosolic DNA and prevents inflammasome activation.

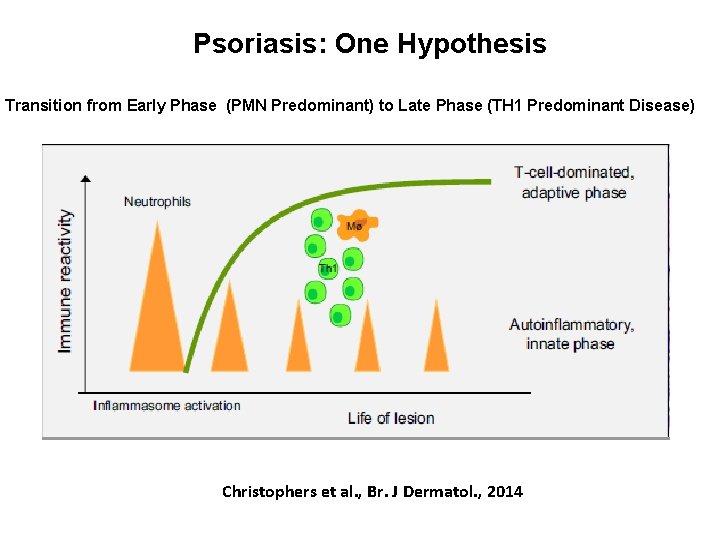
Psoriasis: One Hypothesis Transition from Early Phase (PMN Predominant) to Late Phase (TH 1 Predominant Disease) Christophers et al. , Br. J Dermatol. , 2014

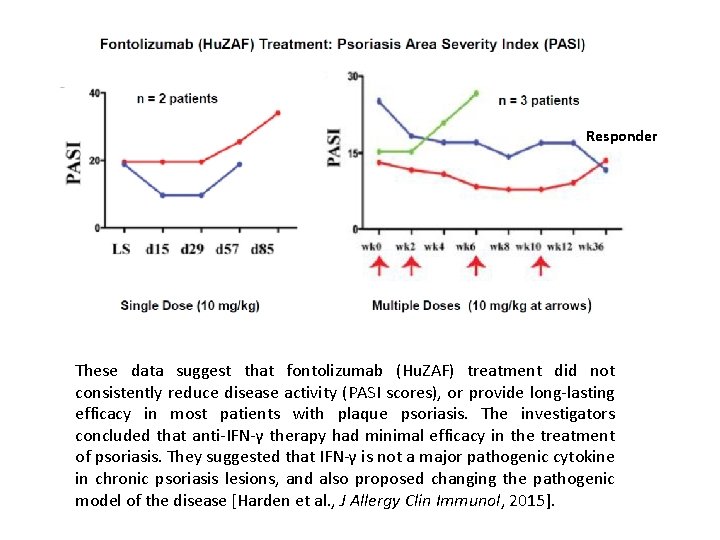
Responder These data suggest that fontolizumab (Hu. ZAF) treatment did not consistently reduce disease activity (PASI scores), or provide long-lasting efficacy in most patients with plaque psoriasis. The investigators concluded that anti-IFN-γ therapy had minimal efficacy in the treatment of psoriasis. They suggested that IFN-γ is not a major pathogenic cytokine in chronic psoriasis lesions, and also proposed changing the pathogenic model of the disease [Harden et al. , J Allergy Clin Immunol, 2015].

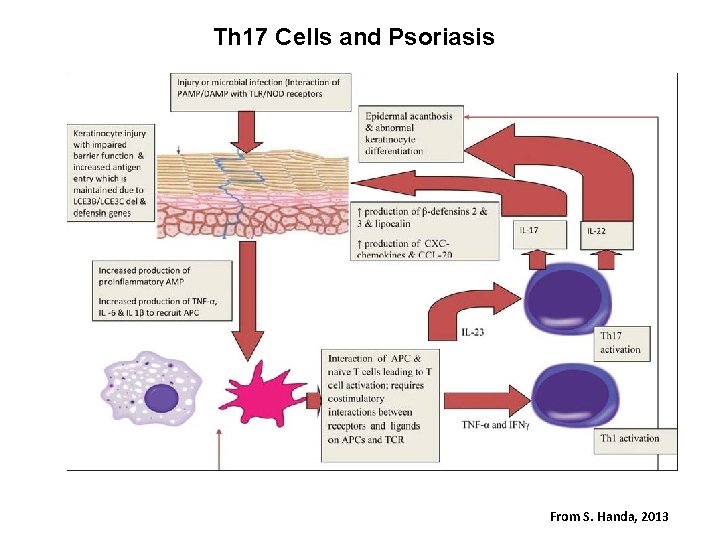
Th 17 Cells and Psoriasis From S. Handa, 2013

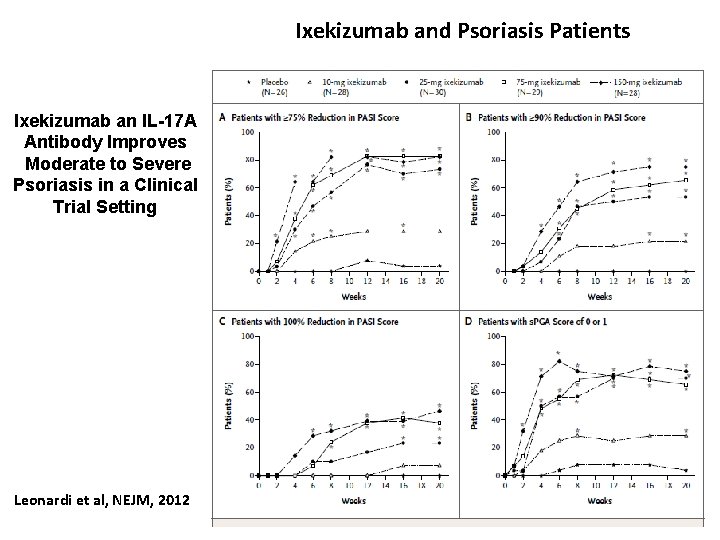
Ixekizumab and Psoriasis Patients Ixekizumab an IL-17 A Antibody Improves Moderate to Severe Psoriasis in a Clinical Trial Setting Leonardi et al, NEJM, 2012

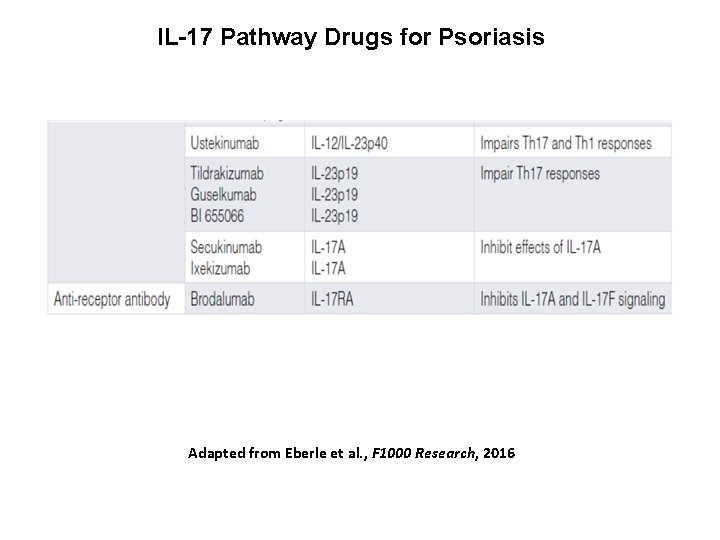
IL-17 Pathway Drugs for Psoriasis Adapted from Eberle et al. , F 1000 Research, 2016

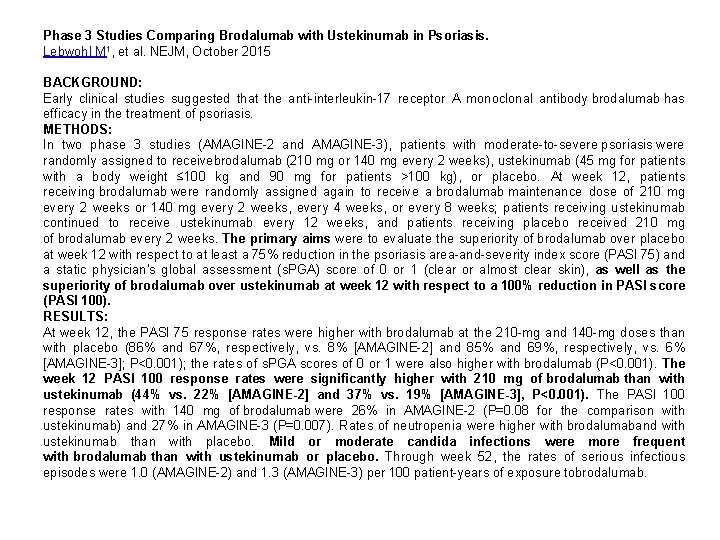
Phase 3 Studies Comparing Brodalumab with Ustekinumab in Psoriasis. Lebwohl M 1, et al. NEJM, October 2015 BACKGROUND: Early clinical studies suggested that the anti-interleukin-17 receptor A monoclonal antibody brodalumab has efficacy in the treatment of psoriasis. METHODS: In two phase 3 studies (AMAGINE-2 and AMAGINE-3), patients with moderate-to-severe psoriasis were randomly assigned to receivebrodalumab (210 mg or 140 mg every 2 weeks), ustekinumab (45 mg for patients with a body weight ≤ 100 kg and 90 mg for patients >100 kg), or placebo. At week 12, patients receiving brodalumab were randomly assigned again to receive a brodalumab maintenance dose of 210 mg every 2 weeks or 140 mg every 2 weeks, every 4 weeks, or every 8 weeks; patients receiving ustekinumab continued to receive ustekinumab every 12 weeks, and patients receiving placebo received 210 mg of brodalumab every 2 weeks. The primary aims were to evaluate the superiority of brodalumab over placebo at week 12 with respect to at least a 75% reduction in the psoriasis area-and-severity index score (PASI 75) and a static physician's global assessment (s. PGA) score of 0 or 1 (clear or almost clear skin), as well as the superiority of brodalumab over ustekinumab at week 12 with respect to a 100% reduction in PASI score (PASI 100). RESULTS: At week 12, the PASI 75 response rates were higher with brodalumab at the 210 -mg and 140 -mg doses than with placebo (86% and 67%, respectively, vs. 8% [AMAGINE-2] and 85% and 69%, respectively, vs. 6% [AMAGINE-3]; P<0. 001); the rates of s. PGA scores of 0 or 1 were also higher with brodalumab (P<0. 001). The week 12 PASI 100 response rates were significantly higher with 210 mg of brodalumab than with ustekinumab (44% vs. 22% [AMAGINE-2] and 37% vs. 19% [AMAGINE-3], P<0. 001). The PASI 100 response rates with 140 mg of brodalumab were 26% in AMAGINE-2 (P=0. 08 for the comparison with ustekinumab) and 27% in AMAGINE-3 (P=0. 007). Rates of neutropenia were higher with brodalumaband with ustekinumab than with placebo. Mild or moderate candida infections were more frequent with brodalumab than with ustekinumab or placebo. Through week 52, the rates of serious infectious episodes were 1. 0 (AMAGINE-2) and 1. 3 (AMAGINE-3) per 100 patient-years of exposure tobrodalumab.

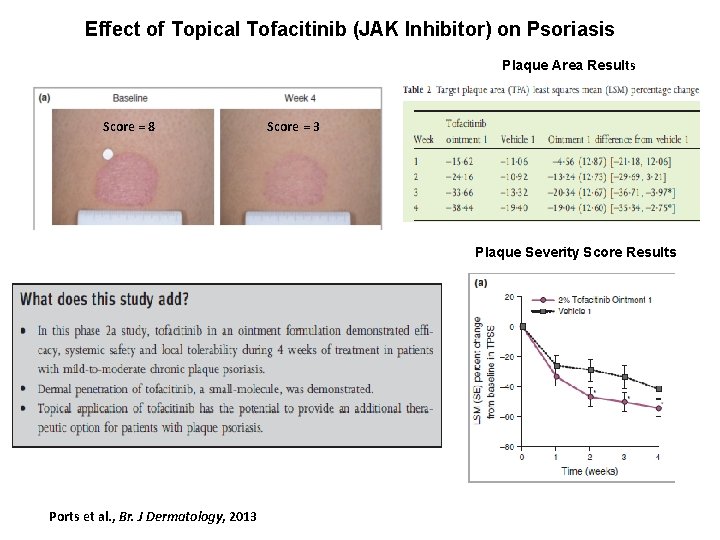
Topical Tofacitinib Treatment for Psoriasis Rejected by FDA , pending more safety data. Milder Disease or Combination Therapy Ports et al. British Journal of Dermatology, 2014

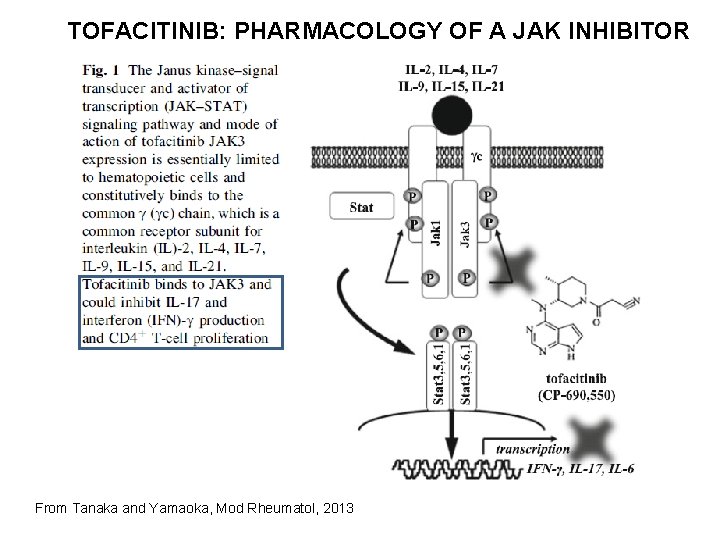
TOFACITINIB: PHARMACOLOGY OF A JAK INHIBITOR From Tanaka and Yamaoka, Mod Rheumatol, 2013

Effect of Topical Tofacitinib (JAK Inhibitor) on Psoriasis Plaque Area Results Score = 8 Score = 3 Plaque Severity Score Results Ports et al. , Br. J Dermatology, 2013

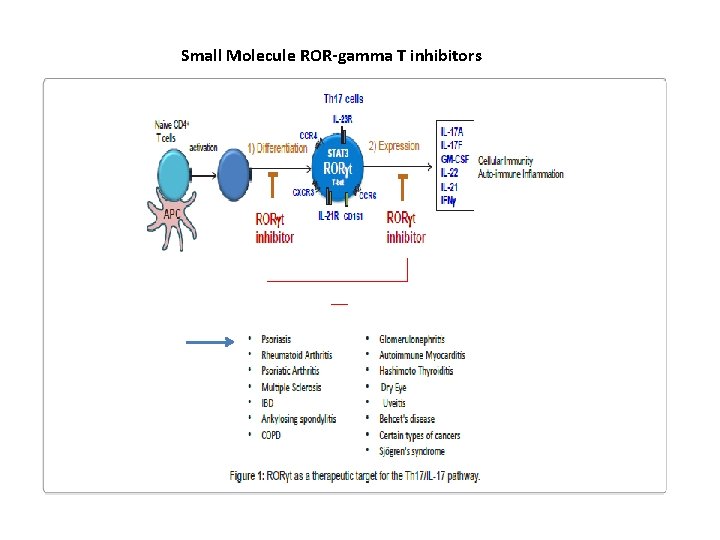
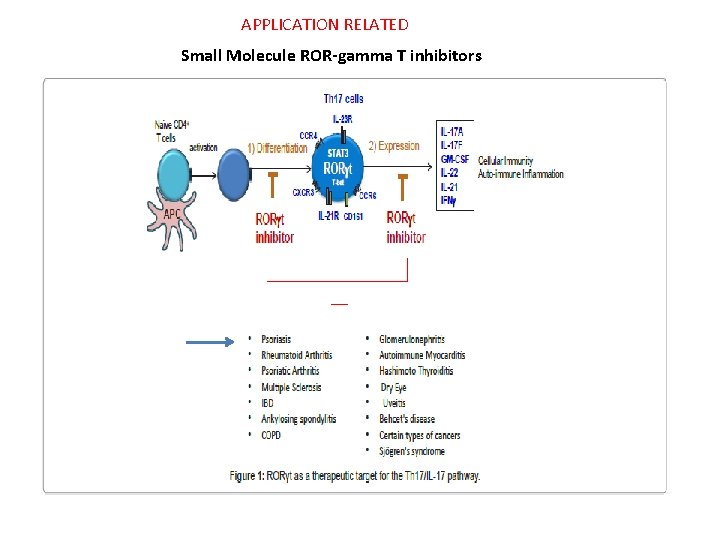
Small Molecule ROR-gamma T inhibitors

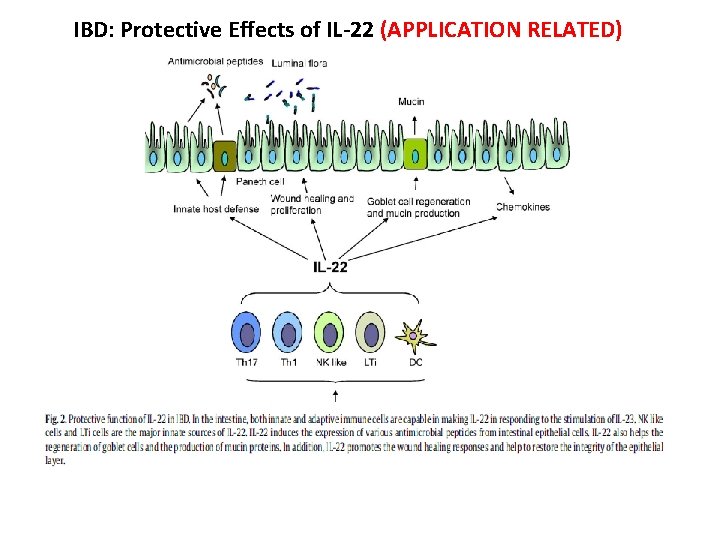
IBD: Protective Effects of IL-22 (APPLICATION RELATED)

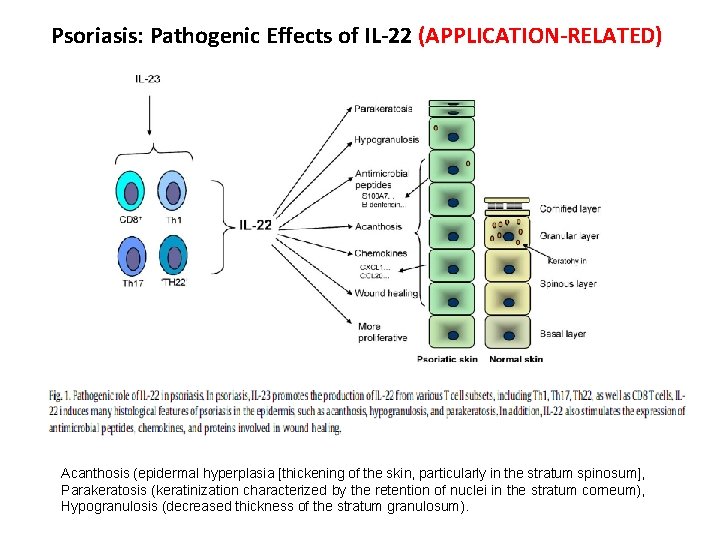
Psoriasis: Pathogenic Effects of IL-22 (APPLICATION-RELATED) Acanthosis (epidermal hyperplasia [thickening of the skin, particularly in the stratum spinosum], Parakeratosis (keratinization characterized by the retention of nuclei in the stratum corneum), Hypogranulosis (decreased thickness of the stratum granulosum).

Application Related IL-17, IL-22 and PSORIASIS Acanthosis (epidermal hyperplasia [thickening of the skin, particularly in the stratum spinosum], Parakeratosis (keratinization characterized by the retention of nuclei in the stratum corneum), and Hypogranulosis (decreased thickness of the stratum granulosum).

APPLICATION RELATED Small Molecule ROR-gamma T inhibitors

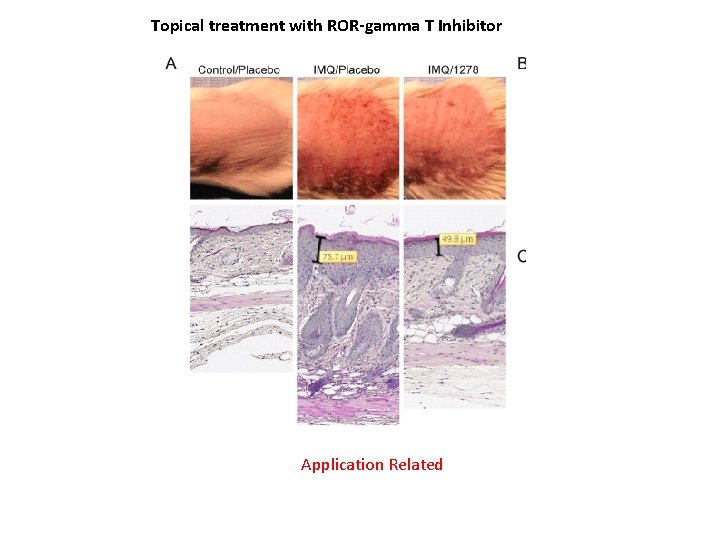
Application Related Systemic treatments aimed at treating inflammation with RORγt inhibition may incur unwanted side effects through cross-reactivity with RORγ. In psoriasis, as with other inflammatory skin disorders, the target tissue is readily accessible. Therefore, local inhibition of RORγ/RORγt with small molecular weight compounds represents a unique opportunity to selectively inhibit aberrant IL-17 cytokine production in the plaque while limiting systemic exposure. In this report (PLOS One, 2016), we describe a novel, potent and highly selective small molecule inhibitor for RORγ/RORγt, that markedly inhibits Th 17 -type cytokine production in multiple assay systems, including (i) in vitro reporter assays, (ii) the in vivo imiquimod mouse model, and (iii) human tissue-based assays, including human peripheral T cells, Th 17 -skewed ex vivo human skin and psoriatic biopsy cultures from psoriasis patients. Based on these supporting data, we are progressing this RORγspecific inverse agonist to clinical trials for topical treatment of mild to moderate psoriasis, expecting that it will impact local cytokine expression and lead to a positive clinical response for patients.

Topical treatment with ROR-gamma T Inhibitor Application Related