PFT Refresher and Modern Inhaler Strategy for COPD





- Slides: 32

PFT Refresher and Modern Inhaler Strategy for COPD Todd C. Hoopman, MD North Idaho Lung, Asthma and Critical Care Coeur d’Alene, ID

Pulmonary Function Testing ▸ Indications: ▸ ▸ ▸ Evaluate: Cough, wheeze, breathlessness, crackles Monitor: COPD, asthma, pulmonary vascular disease Preoperative evaluation: lung resection, abdominal surgery, cardiovascular surgery Surveillance for respiratory complications: CTD or neuromuscular disease Post-lung transplantation ▸ Contraindications: ▸ ▸ ▸ AMI < 30 days Unstable angina Recent eye surgery Current pneumothorax Current tracheostomy Recent thoraco-abdominal surgery

Pulmonary Function Testing ▸ Components: ▸ Spirometry (pre and post-bronchodilator) ▸ Lung volumes evaluation ▸ Diffusion capacity ▸ § § § Other tests: Maximum Voluntary Ventilation Mean Inspiratory Pressure Mean Expiratory Pressure

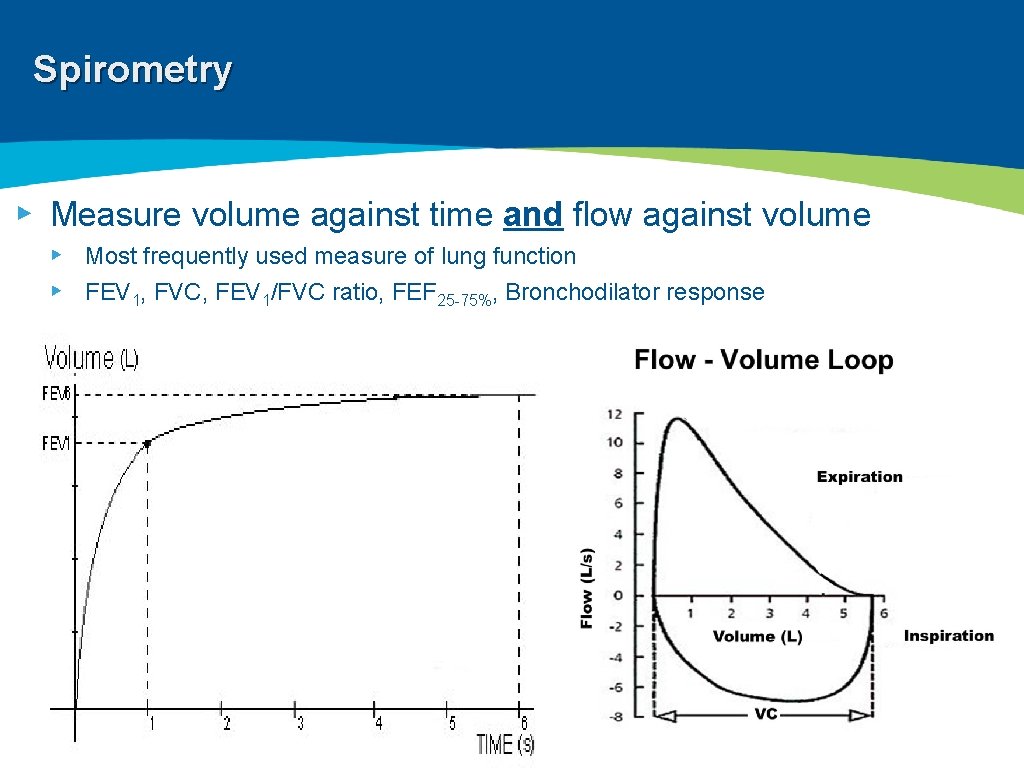
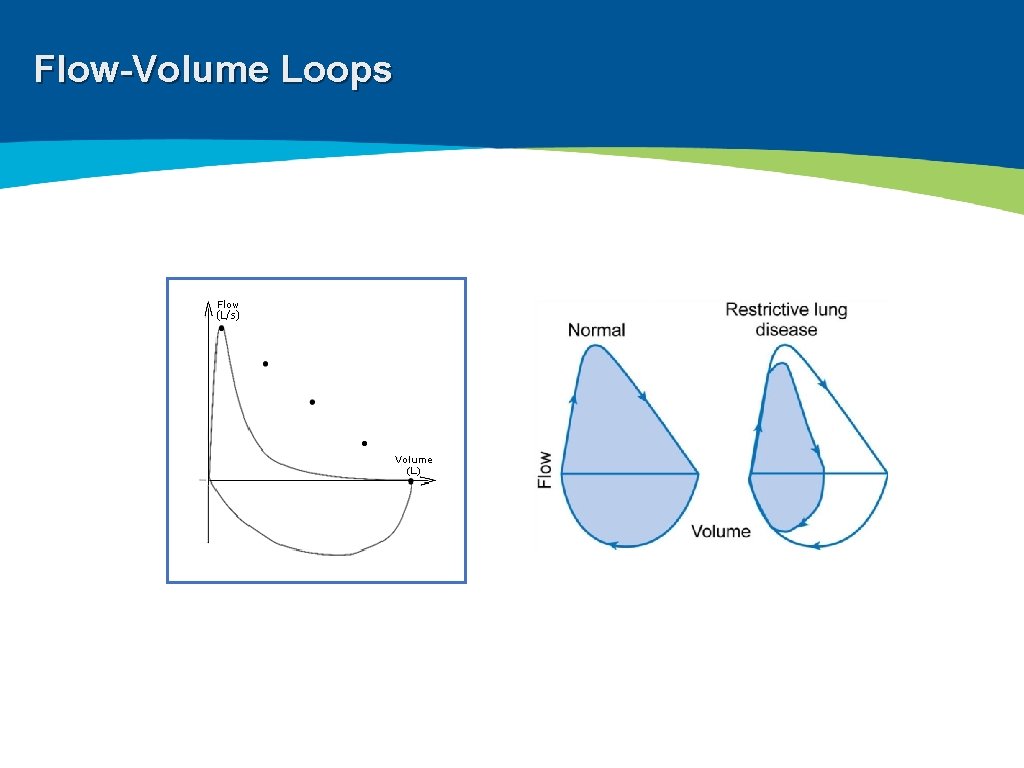
Spirometry ▸ Measure volume against time and flow against volume ▸ Most frequently used measure of lung function ▸ FEV 1, FVC, FEV 1/FVC ratio, FEF 25 -75%, Bronchodilator response

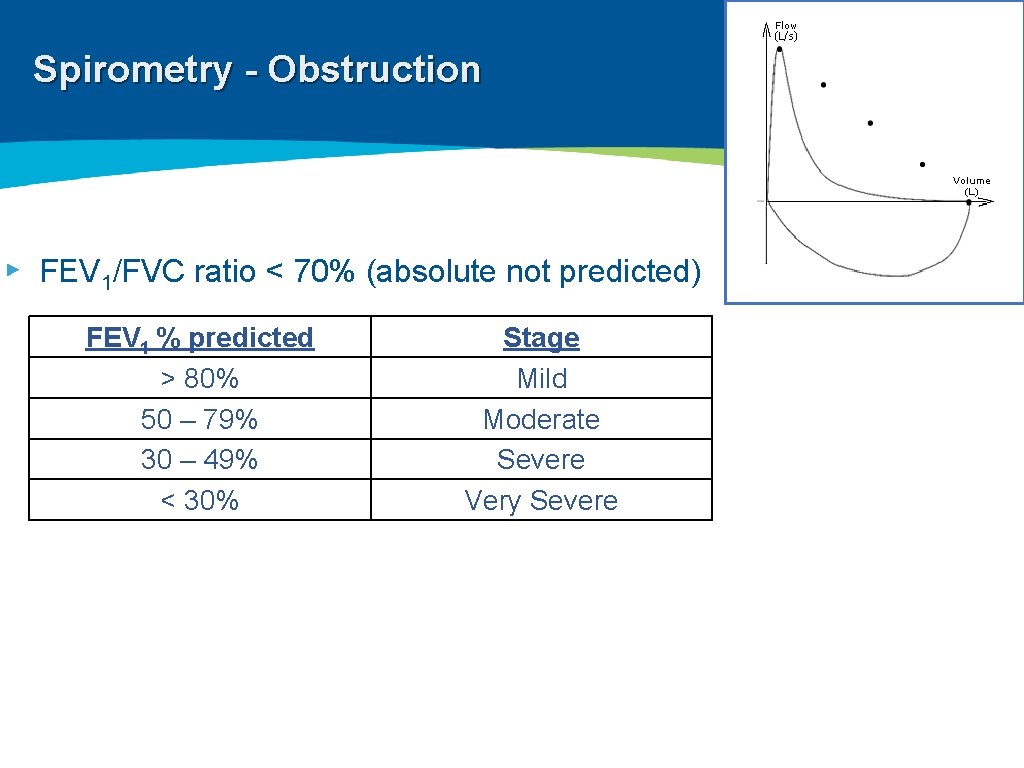
Spirometry - Obstruction ▸ FEV 1/FVC ratio < 70% (absolute not predicted) FEV 1 % predicted > 80% 50 – 79% 30 – 49% < 30% Stage Mild Moderate Severe Very Severe

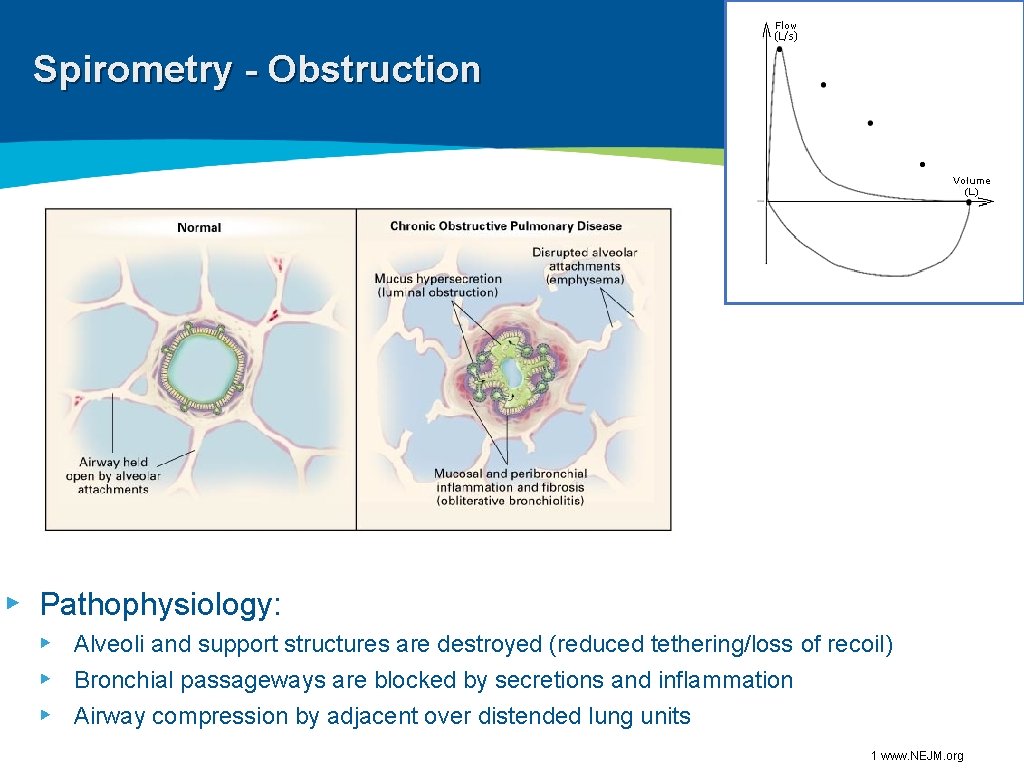
Spirometry - Obstruction ▸ Pathophysiology: ▸ Alveoli and support structures are destroyed (reduced tethering/loss of recoil) ▸ Bronchial passageways are blocked by secretions and inflammation ▸ Airway compression by adjacent over distended lung units 1 www. NEJM. org

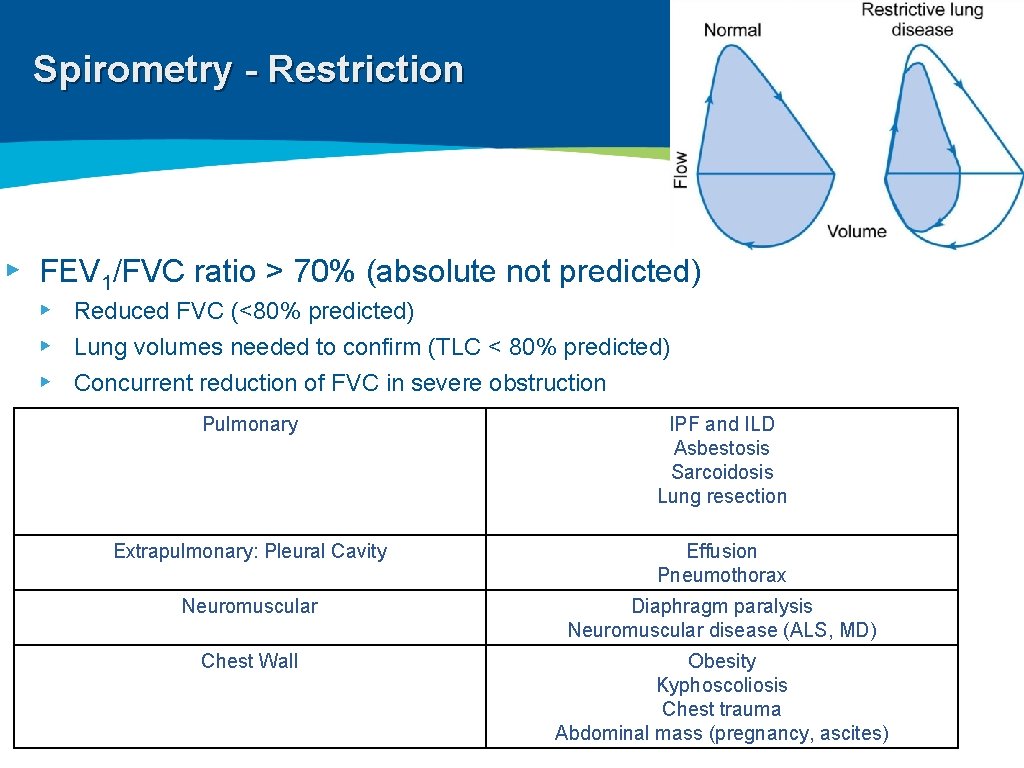
Spirometry - Restriction ▸ FEV 1/FVC ratio > 70% (absolute not predicted) ▸ Reduced FVC (<80% predicted) ▸ Lung volumes needed to confirm (TLC < 80% predicted) ▸ Concurrent reduction of FVC in severe obstruction Pulmonary IPF and ILD Asbestosis Sarcoidosis Lung resection Extrapulmonary: Pleural Cavity Effusion Pneumothorax Neuromuscular Diaphragm paralysis Neuromuscular disease (ALS, MD) Chest Wall Obesity Kyphoscoliosis Chest trauma Abdominal mass (pregnancy, ascites)

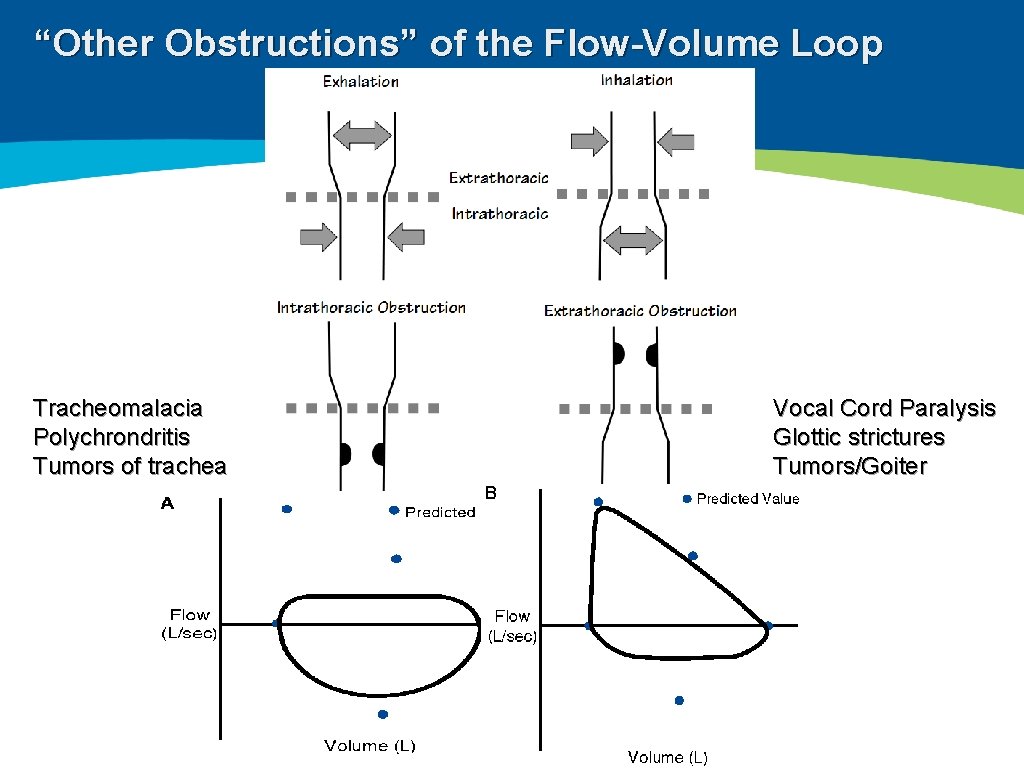
“Other Obstructions” of the Flow-Volume Loop

“Other Obstructions” of the Flow-Volume Loop Tracheomalacia Polychrondritis Tumors of trachea Vocal Cord Paralysis Glottic strictures Tumors/Goiter

Flow-Volume Loops

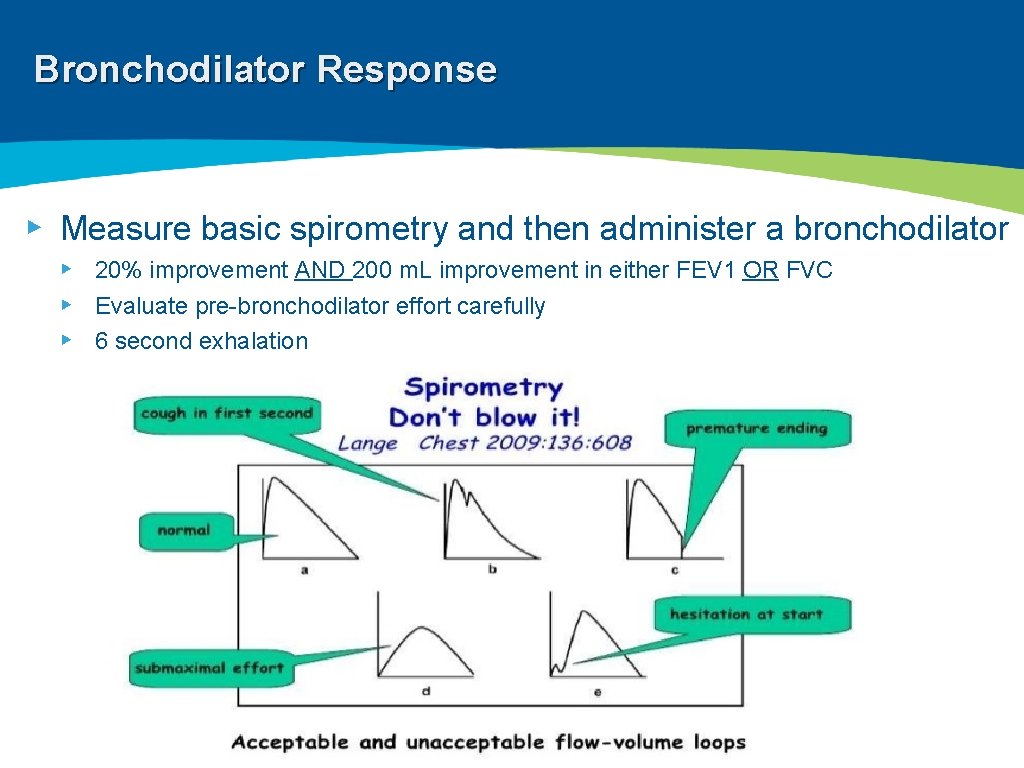
Bronchodilator Response ▸ Measure basic spirometry and then administer a bronchodilator ▸ 20% improvement AND 200 m. L improvement in either FEV 1 OR FVC ▸ Evaluate pre-bronchodilator effort carefully ▸ 6 second exhalation

Lung Volumes ▸ Measure total lung capacity at maximal inspiration ▸ Measure amount of air left in the lungs after maximal expiration (Residual Volume) ▸ *Body plethysmography ▸ Nitrogen washout ▸ Helium washout ▸ Confirm the degree of restriction seen with spirometry ▸ Determine if a reduced Vital Capacity is due to air trapping or intrinsic lung disease

Lung Volumes ▸ Plethysmography can be difficult for some patients: ▸ Morbid obesity ▸ Claustrophobia ▸ Hard of hearing ▸ Mixed disorder ▸ TLC < 80% with FEV 1/FVC < 70% ▸ COPD + IPF ▸ Asthma + Obesity

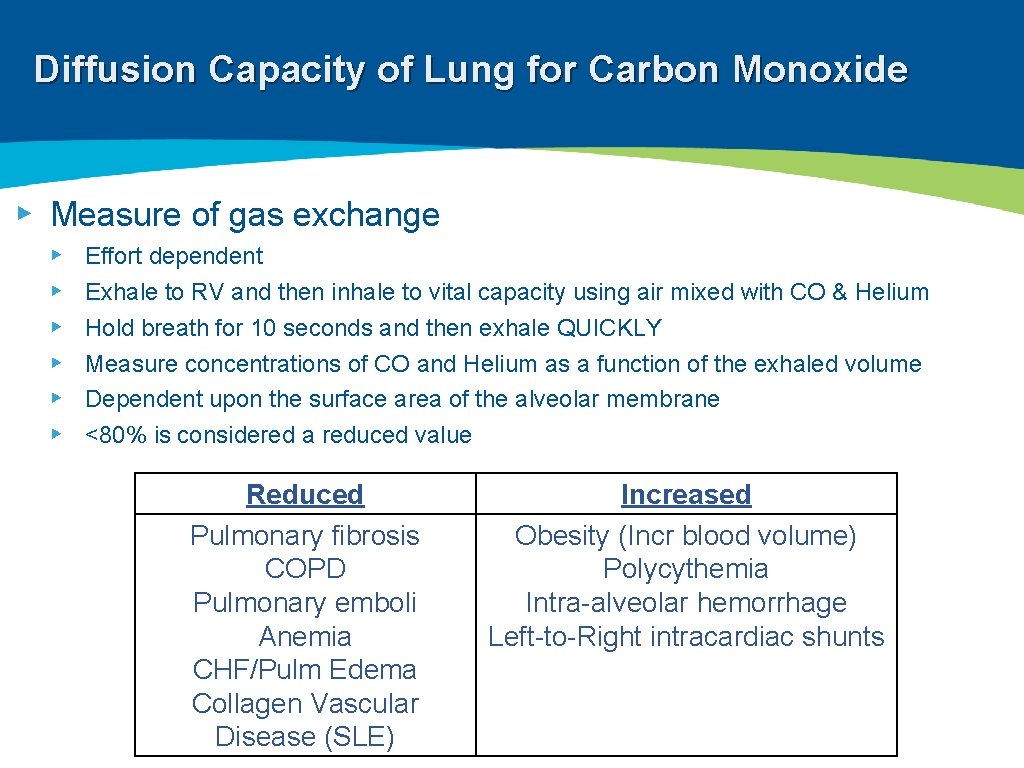
Diffusion Capacity of Lung for Carbon Monoxide ▸ Measure of gas exchange ▸ ▸ ▸ Effort dependent Exhale to RV and then inhale to vital capacity using air mixed with CO & Helium Hold breath for 10 seconds and then exhale QUICKLY Measure concentrations of CO and Helium as a function of the exhaled volume Dependent upon the surface area of the alveolar membrane <80% is considered a reduced value Reduced Pulmonary fibrosis COPD Pulmonary emboli Anemia CHF/Pulm Edema Collagen Vascular Disease (SLE) Increased Obesity (Incr blood volume) Polycythemia Intra-alveolar hemorrhage Left-to-Right intracardiac shunts

COPD Facts 1 ▸ Currently the 4 th leading cause of death in the world 1 ▸ 2012: 3 million deaths ▸ Projected to be the 3 rd leading cause by 2020 ▸ Continued exposure to COPD risk factors and aging of the population ▸ Risk Factors ▸ ▸ ▸ Exposure to noxious particles or gases #1: Tobacco Others: pipe, cigar, water pipe, marijuana Outdoor, occupational and indoor air pollution (burning of biomass fuels) Non-smokers: complex interplay of long-term exposures combined with host factors 1 GOLD 2018 Edition

COPD Facts 1 ▸ Currently the 4 th leading cause of death in the world 1 ▸ 2012: 3 million deaths ▸ Projected to be the 3 rd leading cause by 2020 ▸ Continued exposure to COPD risk factors and aging of the population ▸ Risk Factors ▸ ▸ ▸ Exposure to noxious particles or gases #1: Tobacco Others: pipe, cigar, water pipe, marijuana Outdoor, occupational and indoor air pollution (burning of biomass fuels) Non-smokers: complex interplay of long-term exposures combined with host factors 1 GOLD 2018 Edition

COPD Facts 1 ▸ COPD should be considered in any patient who has dyspnea, chronic cough or sputum production and/or history of exposure to risk factors for the disease ▸ Spirometry is required to make the diagnosis ▸ Post-bronchodilator FEV 1/FVC < 0. 70 confirms the presence of airflow limitation ▸ Key Indicators: ▸ ▸ ▸ Progressive dyspnea. : Worse with exercise & Persistent Chronic cough: May be intermittent and may be non-productive Chronic sputum production History of risk factors: tobacco, biomass fuel burning, occupational Family Hx of COPD and/or childhood factors: low birthweight, childhood resp infxn 1 GOLD 2018 Edition

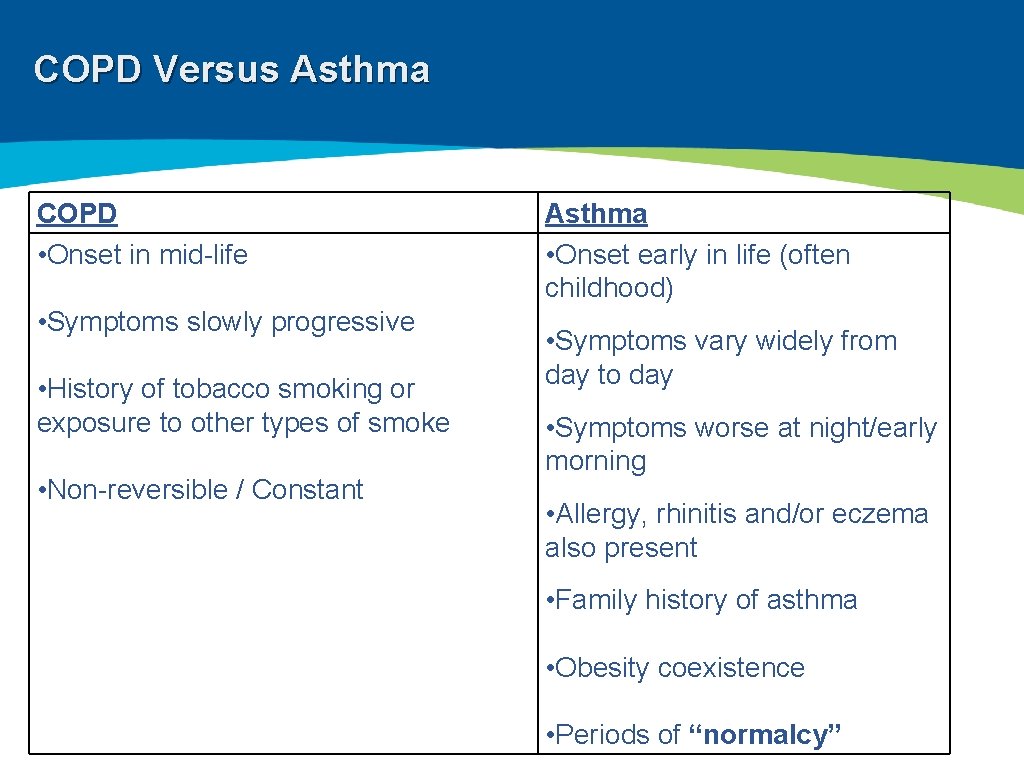
COPD Versus Asthma COPD • Onset in mid-life • Symptoms slowly progressive • History of tobacco smoking or exposure to other types of smoke • Non-reversible / Constant Asthma • Onset early in life (often childhood) • Symptoms vary widely from day to day • Symptoms worse at night/early morning • Allergy, rhinitis and/or eczema also present • Family history of asthma • Obesity coexistence • Periods of “normalcy”

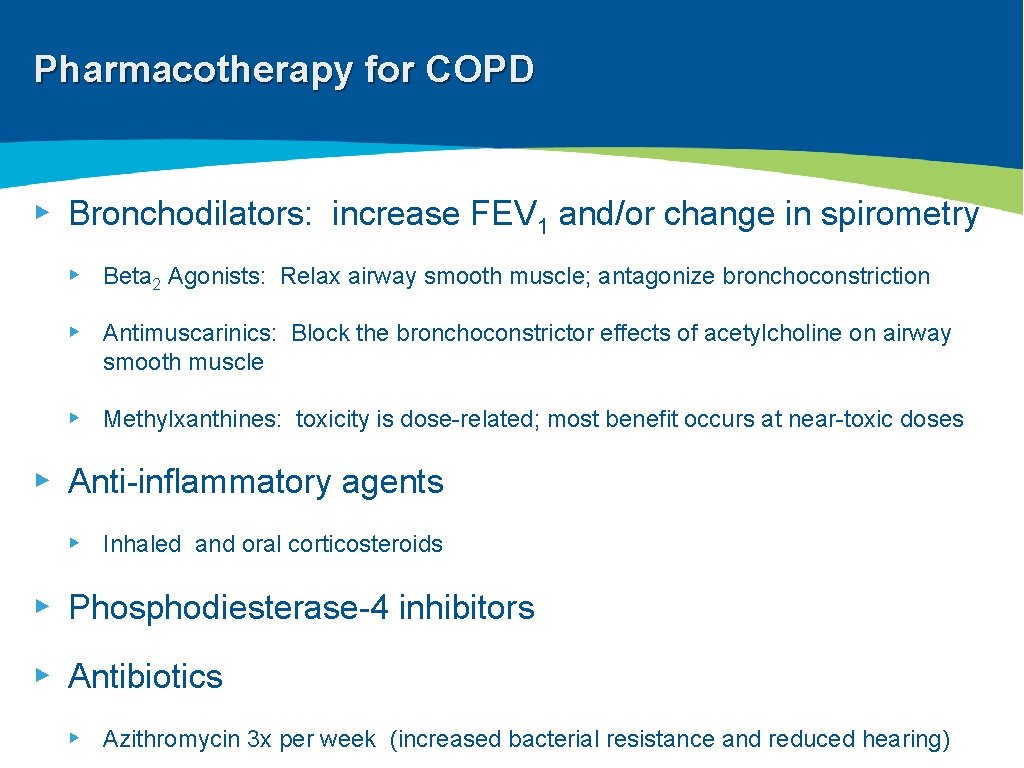
Pharmacotherapy for COPD ▸ Bronchodilators: increase FEV 1 and/or change in spirometry ▸ Beta 2 Agonists: Relax airway smooth muscle; antagonize bronchoconstriction ▸ Antimuscarinics: Block the bronchoconstrictor effects of acetylcholine on airway smooth muscle ▸ Methylxanthines: toxicity is dose-related; most benefit occurs at near-toxic doses ▸ Anti-inflammatory agents ▸ Inhaled and oral corticosteroids ▸ Phosphodiesterase-4 inhibitors ▸ Antibiotics ▸ Azithromycin 3 x per week (increased bacterial resistance and reduced hearing)

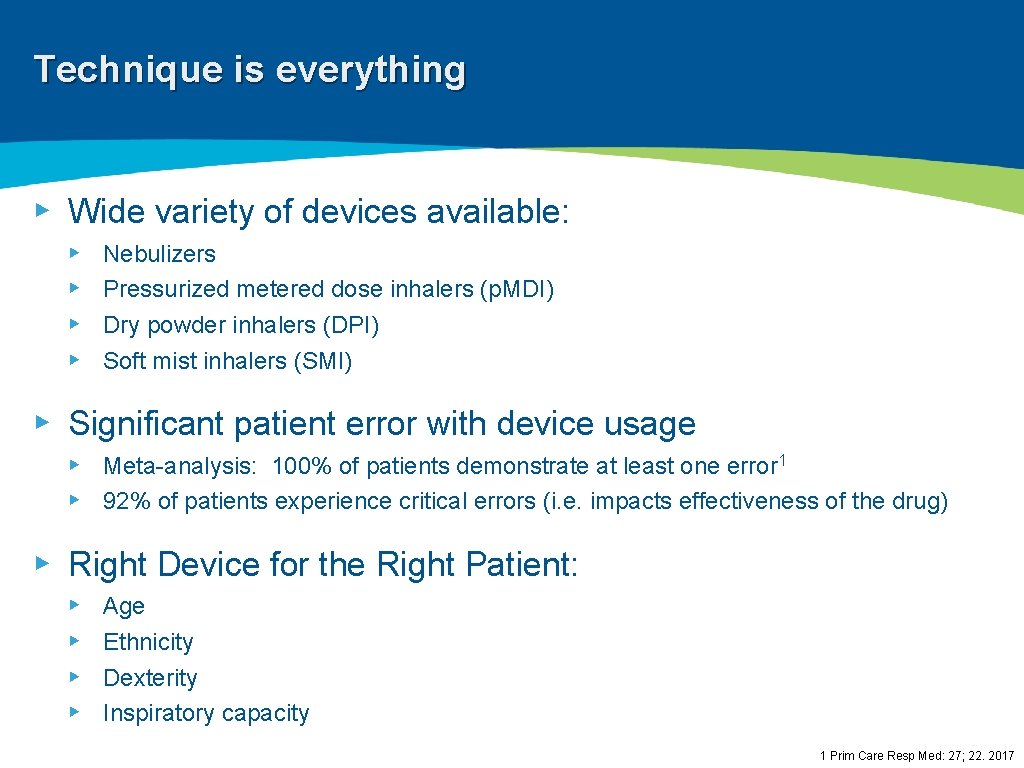
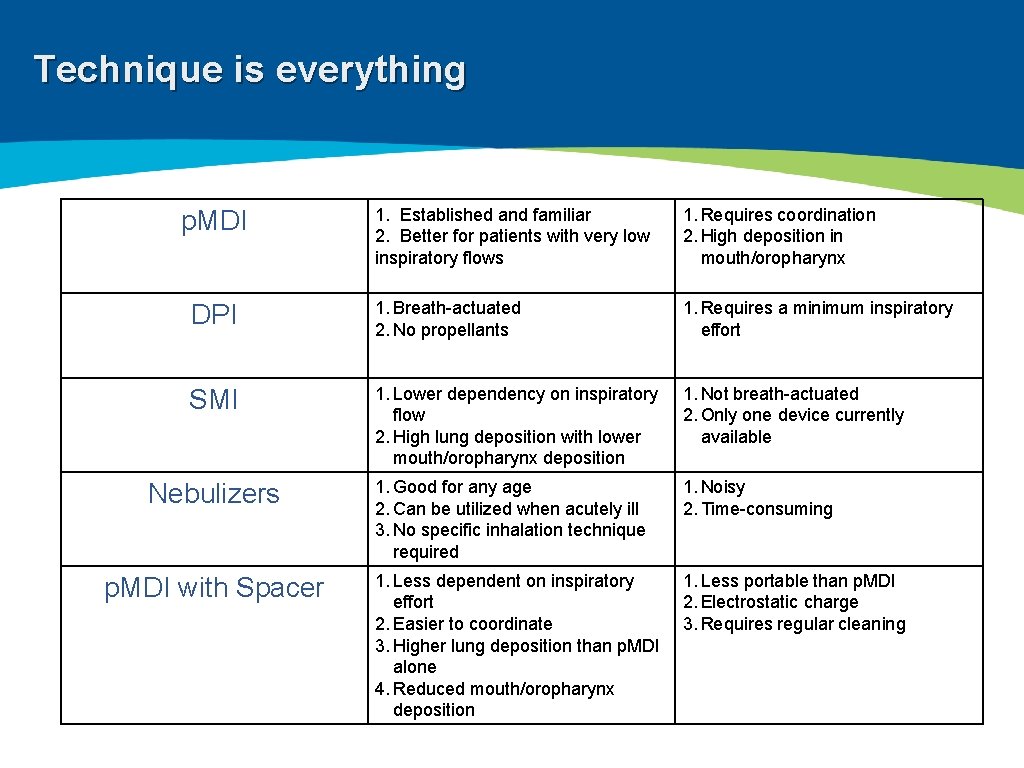
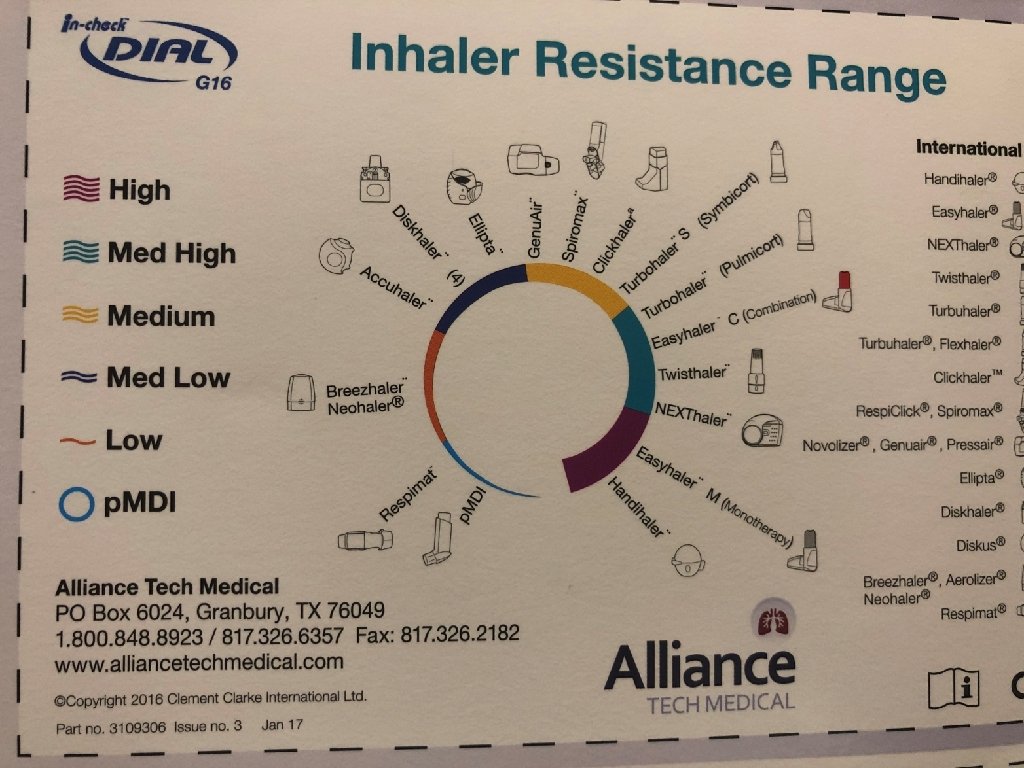
Technique is everything ▸ Wide variety of devices available: ▸ ▸ Nebulizers Pressurized metered dose inhalers (p. MDI) Dry powder inhalers (DPI) Soft mist inhalers (SMI) ▸ Significant patient error with device usage ▸ Meta-analysis: 100% of patients demonstrate at least one error 1 ▸ 92% of patients experience critical errors (i. e. impacts effectiveness of the drug) ▸ Right Device for the Right Patient: ▸ ▸ Age Ethnicity Dexterity Inspiratory capacity 1 Prim Care Resp Med: 27; 22. 2017

Technique is everything 1. Established and familiar 2. Better for patients with very low inspiratory flows 1. Requires coordination 2. High deposition in mouth/oropharynx DPI 1. Breath-actuated 2. No propellants 1. Requires a minimum inspiratory effort SMI 1. Lower dependency on inspiratory flow 2. High lung deposition with lower mouth/oropharynx deposition 1. Not breath-actuated 2. Only one device currently available 1. Good for any age 2. Can be utilized when acutely ill 3. No specific inhalation technique required 1. Noisy 2. Time-consuming 1. Less dependent on inspiratory effort 2. Easier to coordinate 3. Higher lung deposition than p. MDI alone 4. Reduced mouth/oropharynx deposition 1. Less portable than p. MDI 2. Electrostatic charge 3. Requires regular cleaning p. MDI Nebulizers p. MDI with Spacer

Technique is everything 1 Prim Care Resp Med: 27; 22. 2017

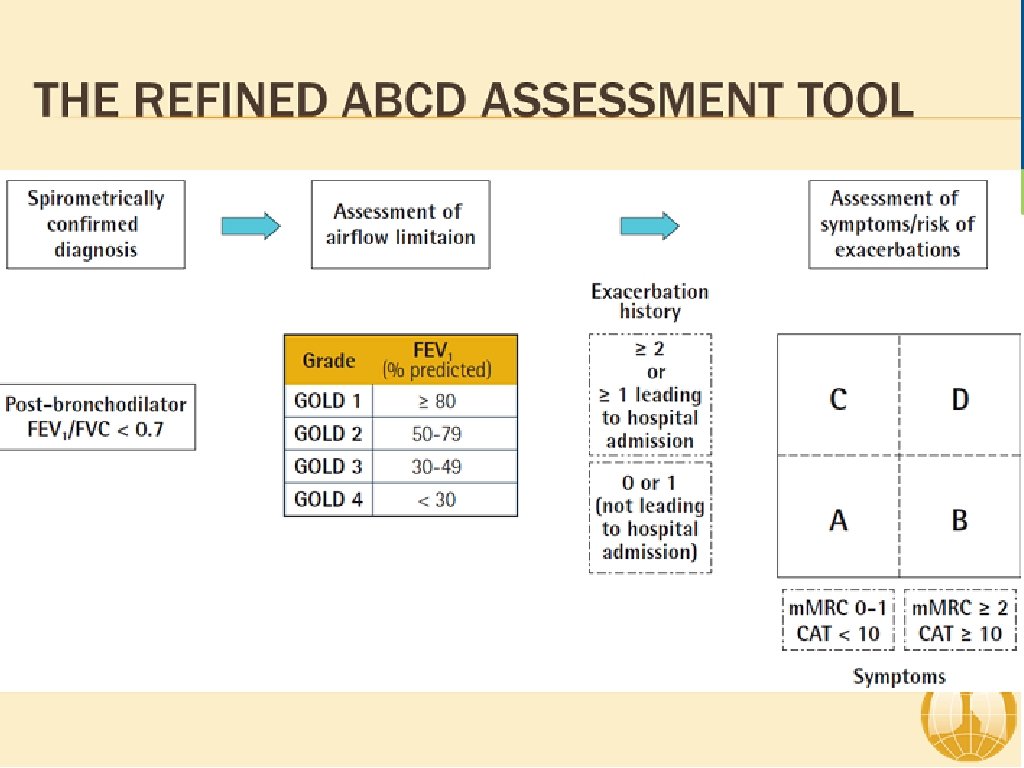
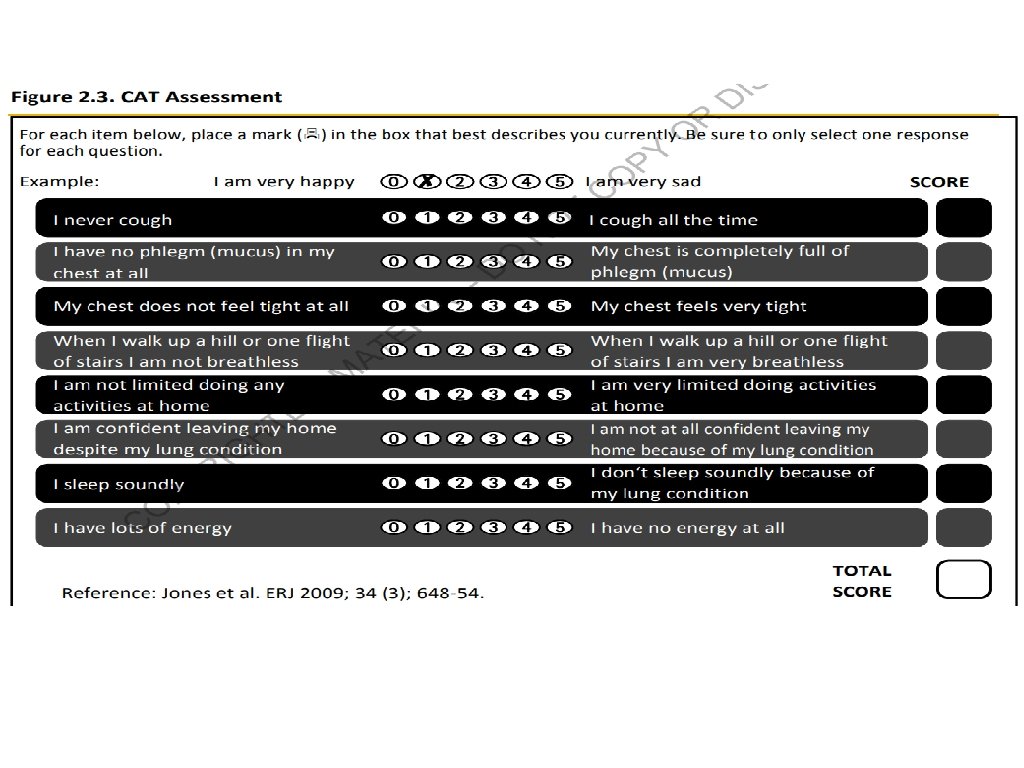
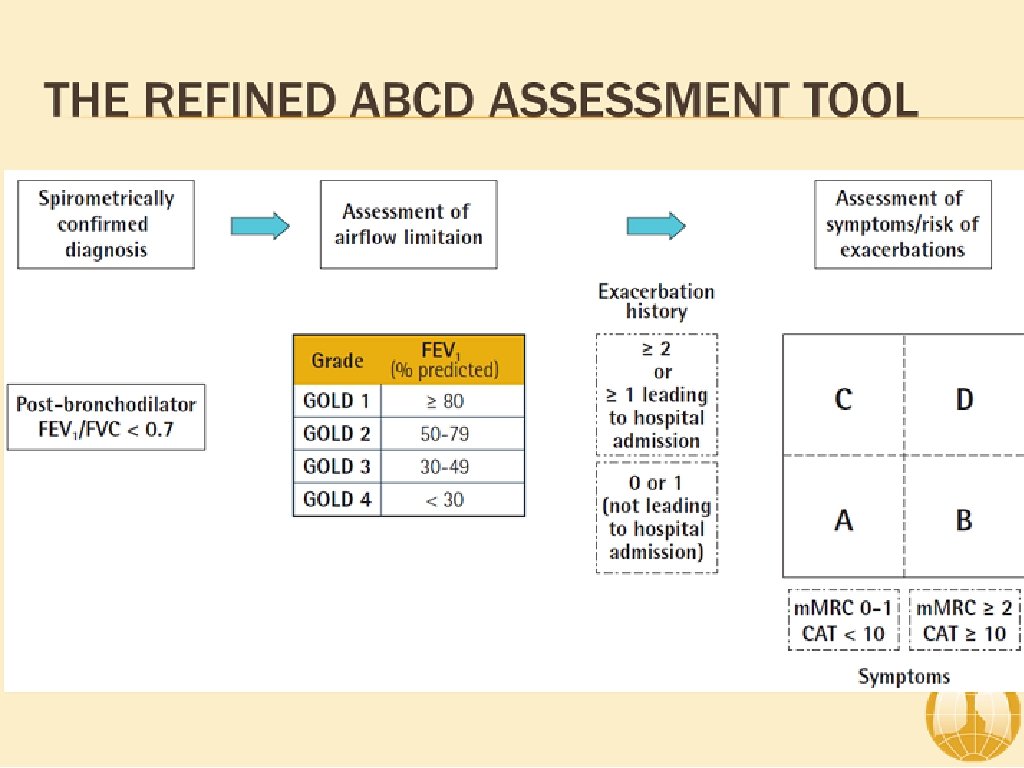
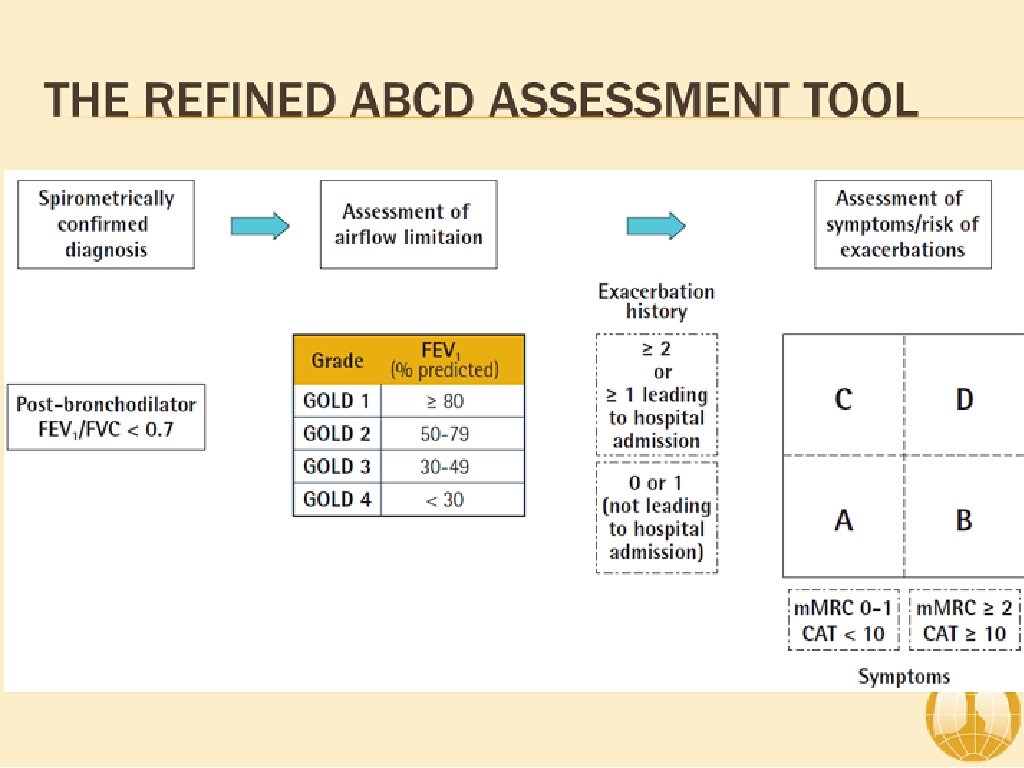
ABCD Assessment Tool of 2011 ▸ Incorporated patient-reported outcomes and highlighted the importance of exacerbation prevention: ▸ No better than spirometry for mortality prediction ▸ Confusion and concerns by this system ▸ Significant patient error with device usage ▸ Meta-analysis: 100% of patients demonstrate at least one error 1 ▸ 92% of patients experience critical errors (i. e. impacts effectiveness of the drug) ▸ Right Device for the Right Patient: ▸ ▸ Age Ethnicity Dexterity Inspiratory capacity



ABCD Assessment Tool of 2011 ▸ Incorporated patient-reported outcomes and highlighted the importance of exacerbation prevention: ▸ No better than spirometry for mortality prediction ▸ Confusion and concerns by this system ▸ Significant patient error with device usage ▸ Meta-analysis: 100% of patients demonstrate at least one error 1 ▸ 92% of patients experience critical errors (i. e. impacts effectiveness of the drug) ▸ Right Device for the Right Patient: ▸ ▸ Age Ethnicity Dexterity Inspiratory capacity

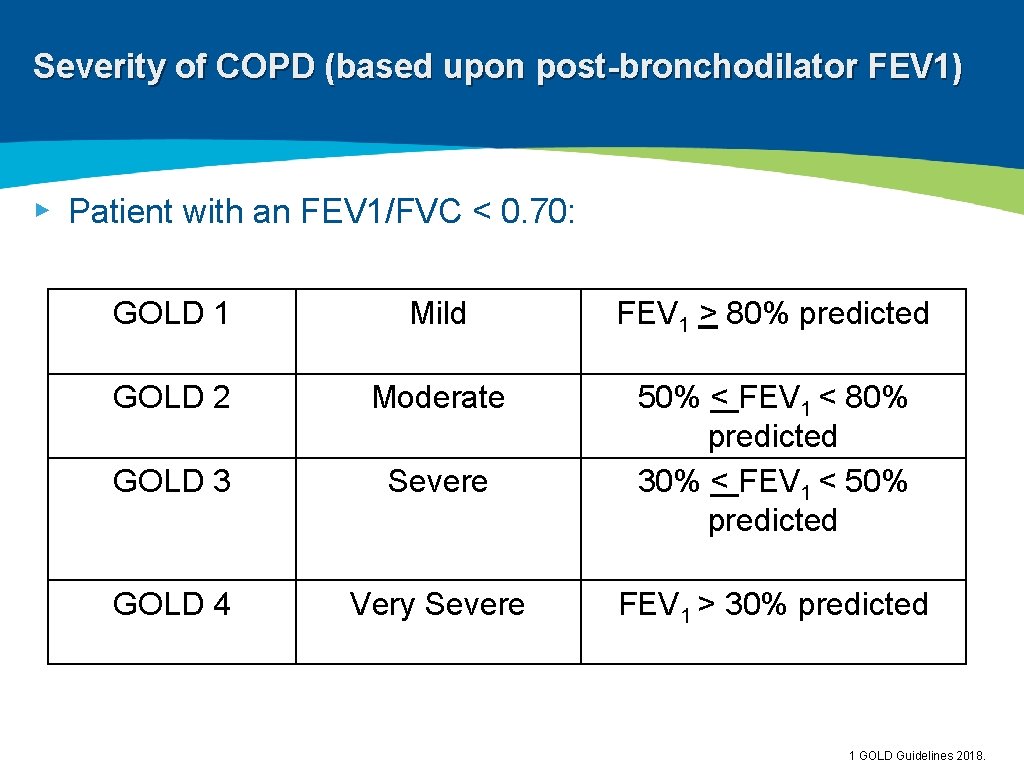
Severity of COPD (based upon post-bronchodilator FEV 1) ▸ Patient with an FEV 1/FVC < 0. 70: GOLD 1 Mild FEV 1 > 80% predicted GOLD 2 Moderate GOLD 3 Severe 50% < FEV 1 < 80% predicted 30% < FEV 1 < 50% predicted GOLD 4 Very Severe FEV 1 > 30% predicted 1 GOLD Guidelines 2018.

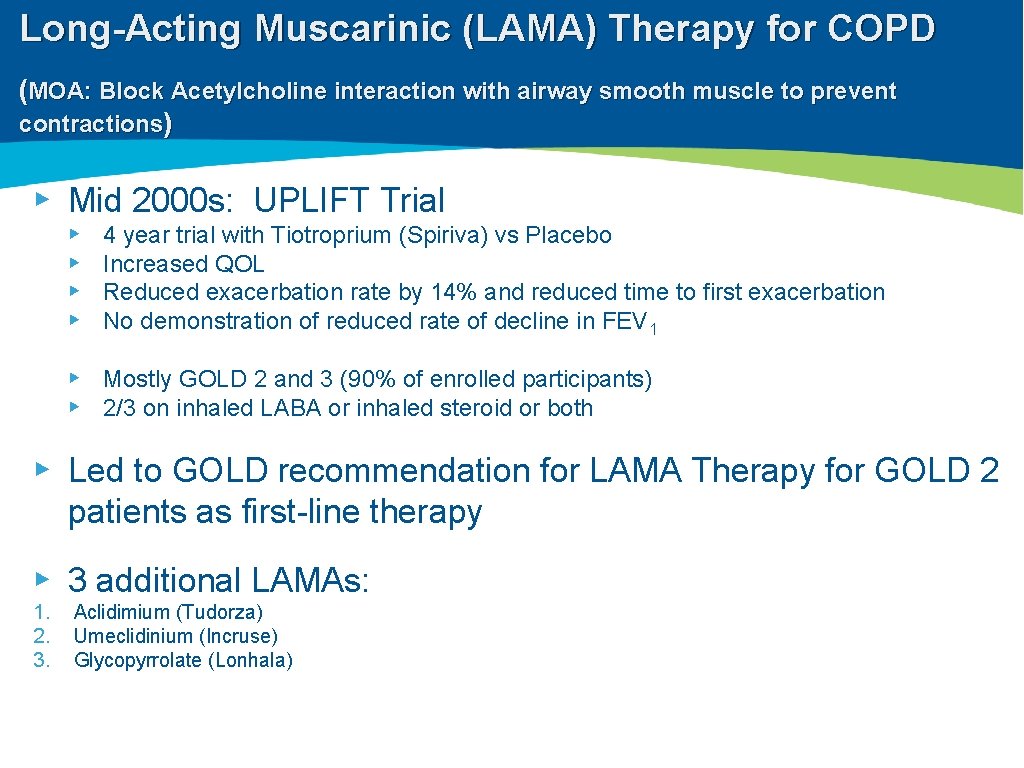
Long-Acting Muscarinic (LAMA) Therapy for COPD (MOA: Block Acetylcholine interaction with airway smooth muscle to prevent contractions) ▸ Mid 2000 s: UPLIFT Trial ▸ ▸ 4 year trial with Tiotroprium (Spiriva) vs Placebo Increased QOL Reduced exacerbation rate by 14% and reduced time to first exacerbation No demonstration of reduced rate of decline in FEV 1 ▸ Mostly GOLD 2 and 3 (90% of enrolled participants) ▸ 2/3 on inhaled LABA or inhaled steroid or both ▸ Led to GOLD recommendation for LAMA Therapy for GOLD 2 patients as first-line therapy ▸ 3 additional LAMAs: 1. 2. 3. Aclidimium (Tudorza) Umeclidinium (Incruse) Glycopyrrolate (Lonhala)

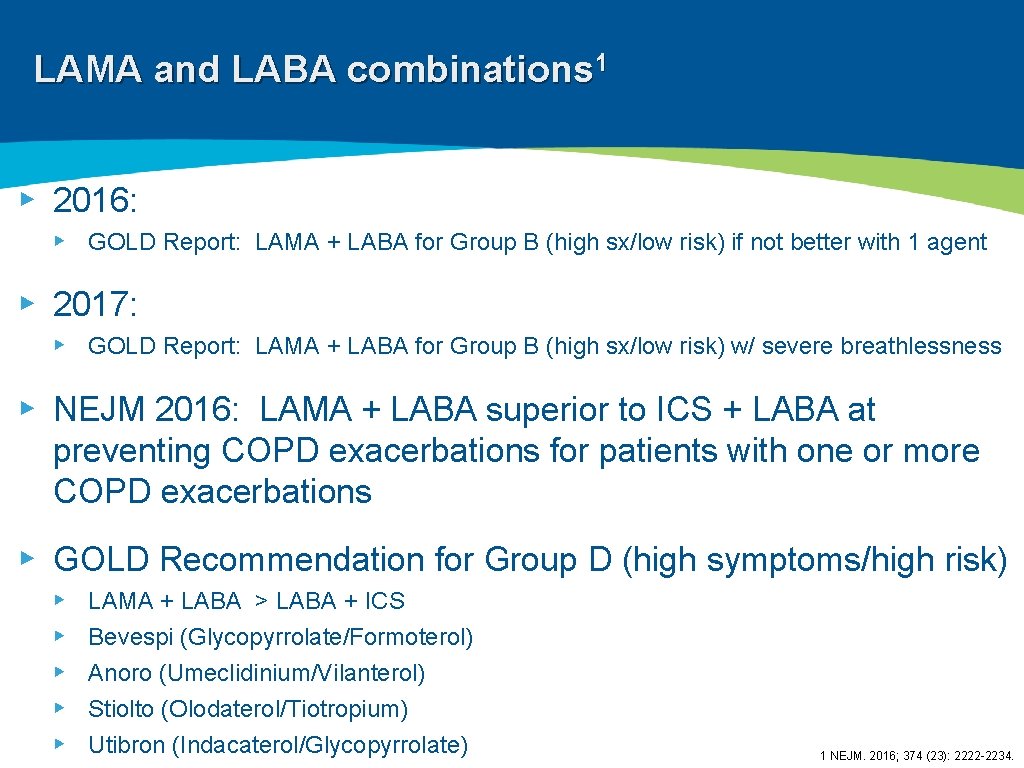
LAMA and LABA combinations 1 ▸ 2016: ▸ GOLD Report: LAMA + LABA for Group B (high sx/low risk) if not better with 1 agent ▸ 2017: ▸ GOLD Report: LAMA + LABA for Group B (high sx/low risk) w/ severe breathlessness ▸ NEJM 2016: LAMA + LABA superior to ICS + LABA at preventing COPD exacerbations for patients with one or more COPD exacerbations ▸ GOLD Recommendation for Group D (high symptoms/high risk) ▸ ▸ ▸ LAMA + LABA > LABA + ICS Bevespi (Glycopyrrolate/Formoterol) Anoro (Umeclidinium/Vilanterol) Stiolto (Olodaterol/Tiotropium) Utibron (Indacaterol/Glycopyrrolate) 1 NEJM. 2016; 374 (23): 2222 -2234.


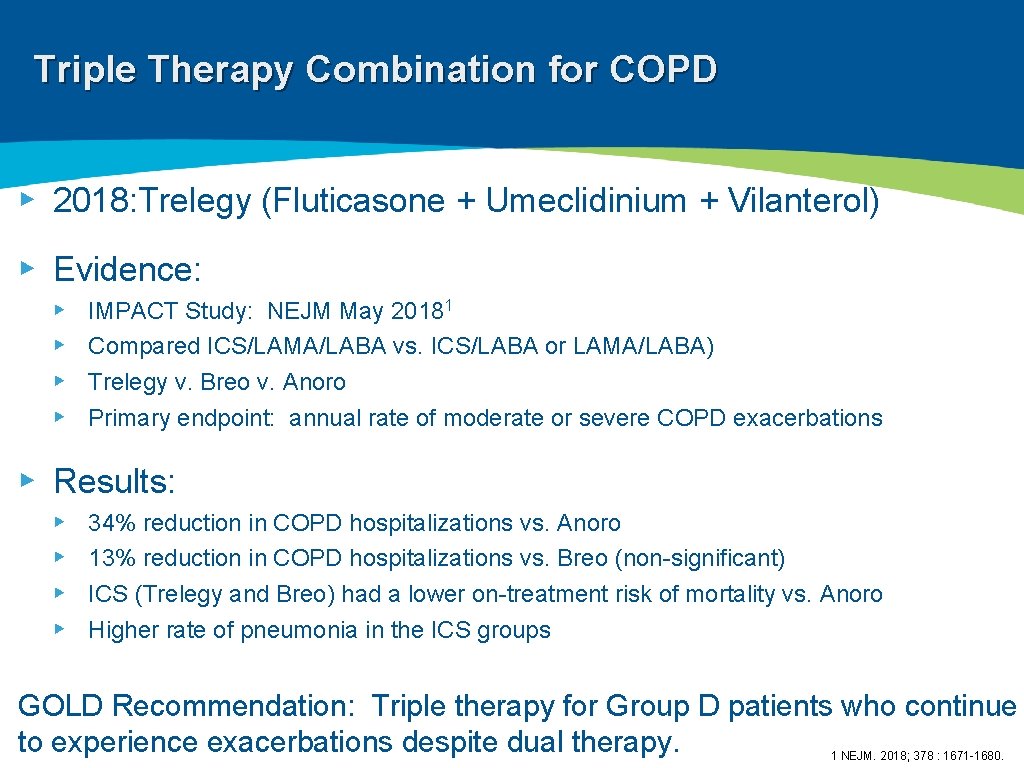
Triple Therapy Combination for COPD ▸ 2018: Trelegy (Fluticasone + Umeclidinium + Vilanterol) ▸ Evidence: ▸ ▸ IMPACT Study: NEJM May 20181 Compared ICS/LAMA/LABA vs. ICS/LABA or LAMA/LABA) Trelegy v. Breo v. Anoro Primary endpoint: annual rate of moderate or severe COPD exacerbations ▸ Results: ▸ ▸ 34% reduction in COPD hospitalizations vs. Anoro 13% reduction in COPD hospitalizations vs. Breo (non-significant) ICS (Trelegy and Breo) had a lower on-treatment risk of mortality vs. Anoro Higher rate of pneumonia in the ICS groups GOLD Recommendation: Triple therapy for Group D patients who continue to experience exacerbations despite dual therapy. 1 NEJM. 2018; 378 : 1671 -1680.

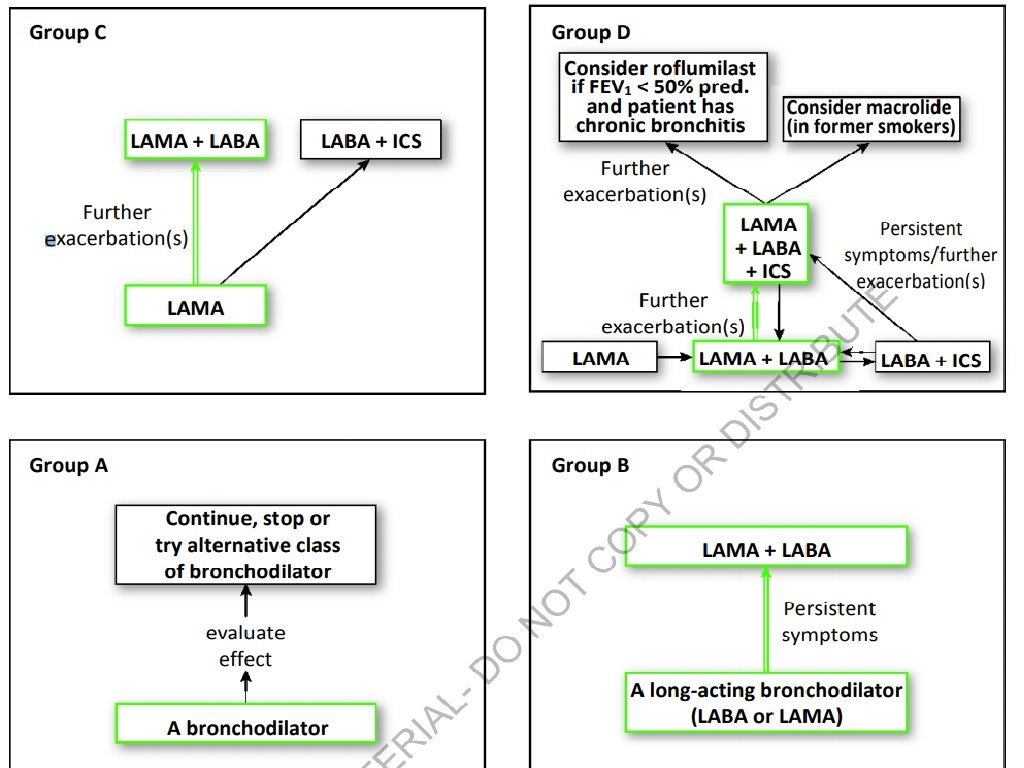
GOLD Recommendations