PFO closures Is there a need Lowell Satler






























- Slides: 41

PFO closures: Is there a need? Lowell Satler, MD Washington Hospital Center

DISCLOSURES Lowell F. Satler, MD Grants/Contracted Research – Medtronic Cardio. Vascular, Inc. , Edwards Lifesciences LLC, Abbott Vascular Speakers Bureau -Daiichi-Sankyo, Inc. -Eli Lilly and Company

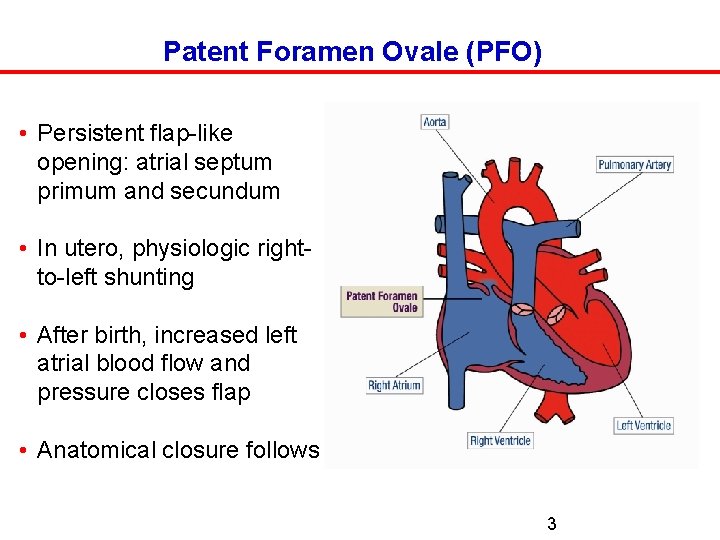
Patent Foramen Ovale (PFO) • Persistent flap-like opening: atrial septum primum and secundum • In utero, physiologic rightto-left shunting • After birth, increased left atrial blood flow and pressure closes flap • Anatomical closure follows 3

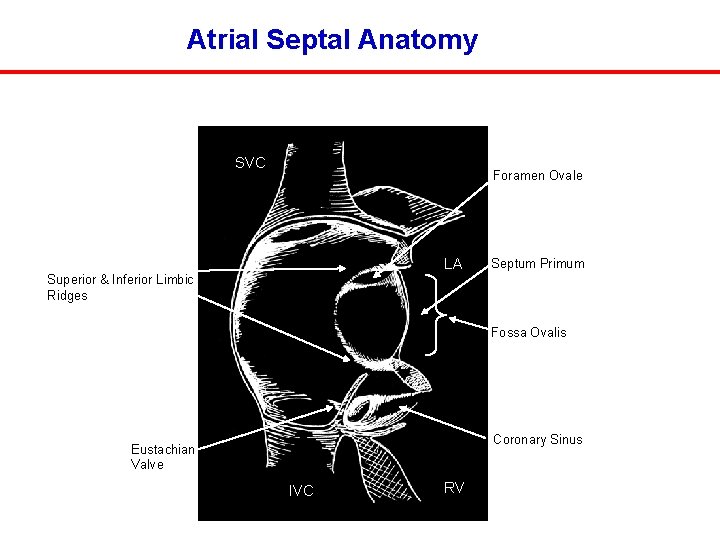
Atrial Septal Anatomy SVC Foramen Ovale LA Septum Primum Superior & Inferior Limbic Ridges Fossa Ovalis Coronary Sinus Eustachian Valve IVC RV

Presumed mechanism of Stroke with PFO Pressure in RA > Pressure in LA: • Early systole • Valsalva • Coughing • Pulmonary hypertension • COPD • Pregnancy • Asthmatics • Wind instruments • Decompression sickness (diving) • High altitude flying • Obstructive sleep patterns PRA RA PLA LA

Role of PFO in Clinical Disease • Cardioembolic stroke • Cryptogenic stroke • Brain abscess • Platypnea-orthodeoxia • Migraine with aura • Decompression illness ischemia/stroke • Wind instrument

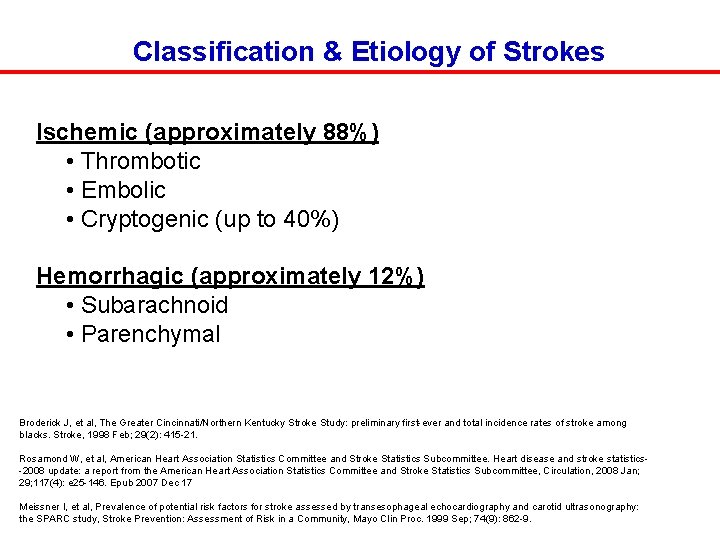
Classification & Etiology of Strokes Ischemic (approximately 88%) • Thrombotic • Embolic • Cryptogenic (up to 40%) Hemorrhagic (approximately 12%) • Subarachnoid • Parenchymal Broderick J, et al, The Greater Cincinnati/Northern Kentucky Stroke Study: preliminary first-ever and total incidence rates of stroke among blacks. Stroke, 1998 Feb; 29(2): 415 -21. Rosamond W, et al, American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee, Circulation, 2008 Jan; 29; 117(4): e 25 -146. Epub 2007 Dec 17 Meissner I, et al, Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC study, Stroke Prevention: Assessment of Risk in a Community, Mayo Clin Proc. 1999 Sep; 74(9): 862 -9.

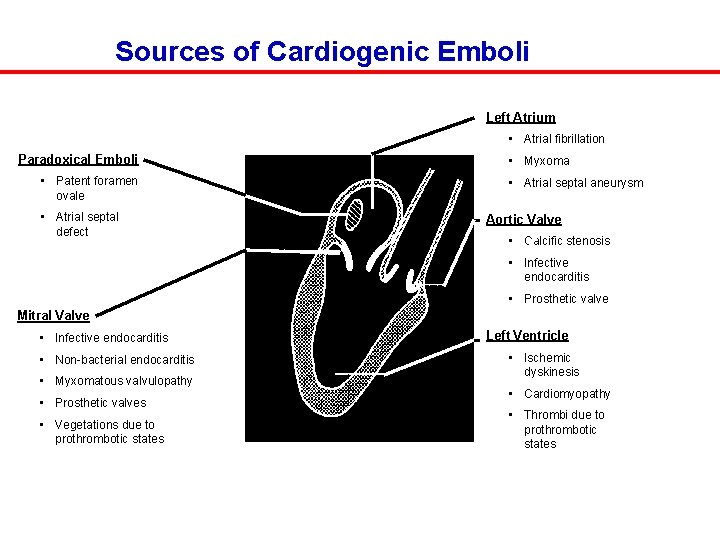
Sources of Cardiogenic Emboli Left Atrium • Atrial fibrillation Paradoxical Emboli • Patent foramen ovale • Atrial septal defect • Myxoma • Atrial septal aneurysm Aortic Valve • Calcific stenosis • Infective endocarditis • Prosthetic valve Mitral Valve • Infective endocarditis • Non-bacterial endocarditis • Myxomatous valvulopathy • Prosthetic valves • Vegetations due to prothrombotic states Left Ventricle • Ischemic dyskinesis • Cardiomyopathy • Thrombi due to prothrombotic states

Cryptogenic Stroke • 700, 000 strokes/yr in US • 80 -85% ischemic • 30 -40% of strokes remain defined as cryptogenic • 40 -60% frequency of PFO among cryptogenic strokes • ~100, 000 strokes/yr with PFO as only identified potential etiology Kim D, Saver JL. Reviews in Neurological Diseases 2005; 2(1): 1 -7

Implication of PFO in Cryptogenic Stroke Prevalence of PFO in “Normal” Population: 10 -25% Lechat, et al - NEJM 5/88 Webster, et al - Lancet 1988 Mayo Autopsy Study Prevalence in Stroke Population <60 y. o. : 40 -50% Ranous, Mas, et al - STROKE 1/93 Webster, et al - Lancet 1988 Lausanne Study - Neurology 5/96 Lechat, et al - NEJM 5/88

Case 1 • 55 year old man • Hx: AAA, smoker, HTN, chol, achilles repair 1 week PTA • Acute left motor deficits, dysarthria over 2 hours • Outside ER: CT, labs, EKG, US, ECHO, leg doppler • Outside Diagnosis: TIA

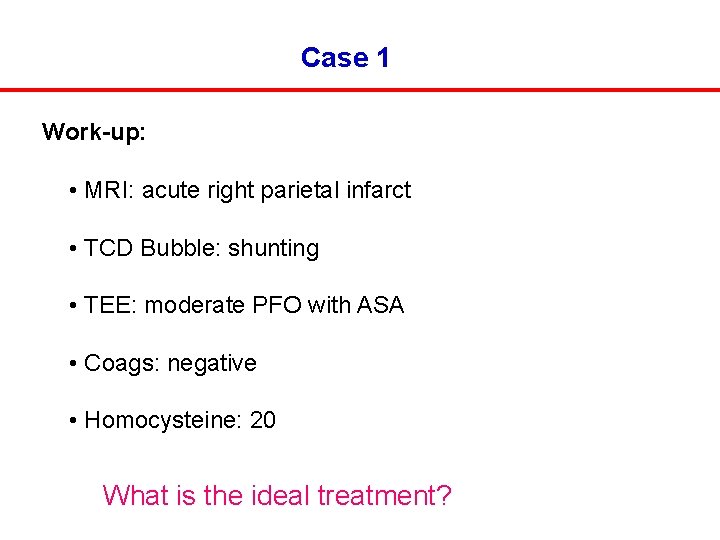
Case 1 Work-up: • MRI: acute right parietal infarct • TCD Bubble: shunting • TEE: moderate PFO with ASA • Coags: negative • Homocysteine: 20 What is the ideal treatment?

Case 2 • 50 year old medical school professor • No PMH or vascular risk factors • Event: • During 9 hour air travel, reads book, does not get up. • After flight, enters terminal walking to customs. Has vigorous sneezing. • Within minutes becomes confused and staggers to floor, but recovers in minutes. • Hospitalized.

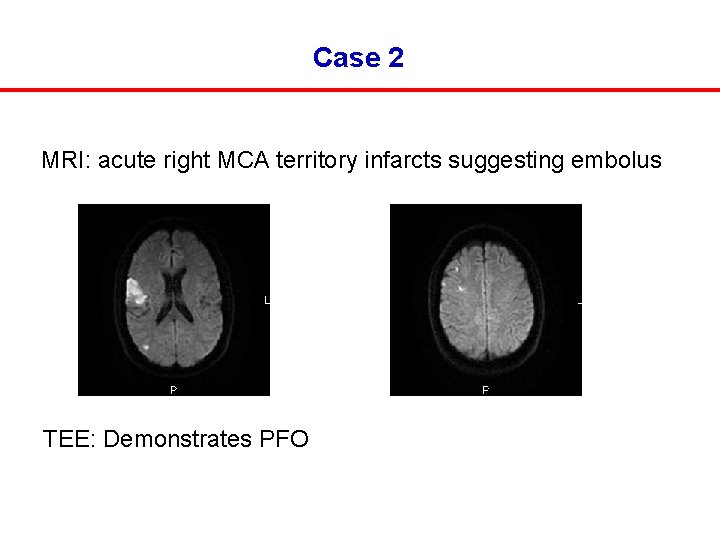
Case 2 MRI: acute right MCA territory infarcts suggesting embolus TEE: Demonstrates PFO

Case 2 Work-up: • normal CTA head and neck, routine ECHO, BP, coagulation labs, extremity dopplers • ECHO with bubble reveals large PFO with shunting at valsalva Diagnosis: • Presumed extremity/pelvic vein clot related to stasis (prolonged sit during air travel) with paradoxical embolus after mobilizing clot (walking) and powerful valsalva (sneeze) What is the ideal treatment?

PFO: AAN Practice Guidelines 2004 • There is insufficient evidence of superiority of aspirin or warfarin • Risks of minor bleeding appear greater with warfarin • There is insufficient evidence to evaluate efficacy of surgical or endovascular closure Messe, et al, Neurology 2004; 62, 1042 -1050

AHA-ASA 2006 Secondary Prevention Guidelines • Insufficient data exist to make a recommendation about PFO closure in patients with a first stroke and a PFO.

To Close or Not to Close The RESPECT PFO Clinical Trial is designed to help answer this question.

Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment IDE #G 990318

Clinical Trial Design • The RESPECT PFO Clinical Trial is a randomized evaluation comparing device closure plus medical therapy to current medical therapy standard of care • Maximum 900 patients (450 per arm) • Maximum 75 participating institutions across the U. S.

Clinical Trial Design Overview Subjects: • Recent cryptogenic stroke • 18 -60 years of age Treatment: • PFO device closure versus medical therapy Endpoints: • Stroke or Death

Randomization Groups • Device closure plus medical therapy will include the AMPLATZER PFO Occluder and clopidogrel for one month and aspirin for six months • Medical therapy current standard of care will include one of the four following treatments: • Aspirin alone • Clopidogrel alone dipyridamole • Warfarin alone • Aspirin in combination with

Study Objective To investigate whether percutaneous PFO closure is superior to current standard of care medical treatment in the prevention of recurrent embolic stroke. Selection Criteria Patients with PFO who have had a cryptogenic stroke due to presumed paradoxical embolism within the last 270 days.

Stroke - Definition Acute focal neurological deficit presumed to be due to focal ischemia, and either: 1) Symptoms persisting 24 hours or greater, or 2) Symptoms persisting less than 24 hours but associated with MR or CT findings of a new, neuroanatomically relevant, cerebral infarct

Study Endpoints Primary Endpoint: • Recurrence of a Nonfatal Stroke, Post-randomization Death, or Fatal Ischemic Stroke

Study Endpoints Secondary Endpoints: • Complete closure of the defect demonstrated by TEE and bubble study at 6 -month follow-up (device group) • Absence of recurrent symptomatic cryptogenic nonfatal stroke or cardiovascular death • Absence of TIA

Required Baseline Testing Medical History: Exclusion Criteria • Age <18 years or >60 years • Active endocarditis or other untreated infections • Acute or recent (within 6 months) MI or unstable angina • Organ failure (kidney, liver or lung) • Uncontrolled hypertension (>160/90 mm. Hg) • Uncontrolled diabetes mellitus

Required Baseline Testing Coagulation: • A hypercoagulation panel sent to a core lab Exclusion Criteria • Patients who test positive for Anticardiolipin Ab of the Ig. G or Ig. M, Lupus Anticoagulant, B 2 -glycoprotein-1 antibodies or persistently elevated plasma Homocysteine despite medical therapy.

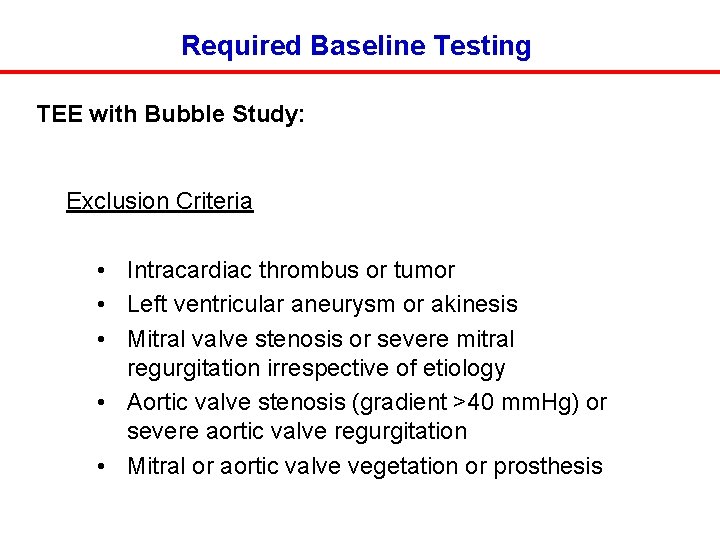
Required Baseline Testing TEE with Bubble Study: Exclusion Criteria • Intracardiac thrombus or tumor • Left ventricular aneurysm or akinesis • Mitral valve stenosis or severe mitral regurgitation irrespective of etiology • Aortic valve stenosis (gradient >40 mm. Hg) or severe aortic valve regurgitation • Mitral or aortic valve vegetation or prosthesis

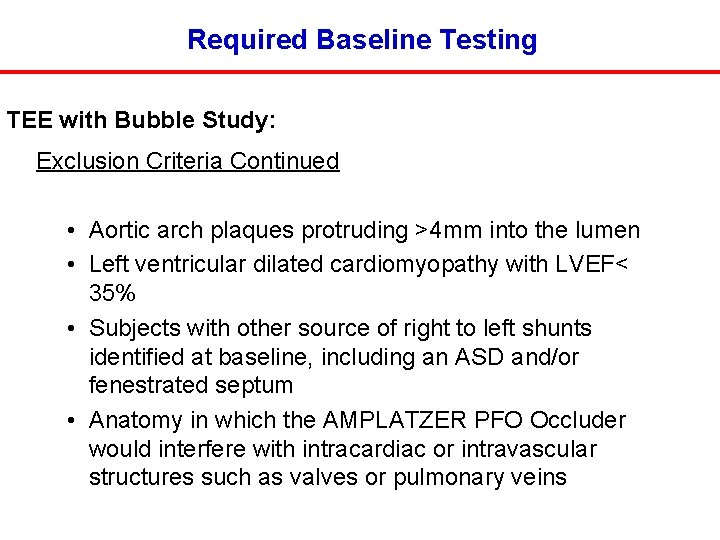
Required Baseline Testing TEE with Bubble Study: Exclusion Criteria Continued • Aortic arch plaques protruding >4 mm into the lumen • Left ventricular dilated cardiomyopathy with LVEF< 35% • Subjects with other source of right to left shunts identified at baseline, including an ASD and/or fenestrated septum • Anatomy in which the AMPLATZER PFO Occluder would interfere with intracardiac or intravascular structures such as valves or pulmonary veins

Required Baseline Testing ECG or Holter Exclusion Criteria: • Atrial fibrillation/atrial flutter (chronic or intermittent) Pregnancy Test Exclusion Criteria: • Pregnant or desire to become pregnant within the next year 33

Device Description • Self Expandable • Nitinol wire 0. 005 - 0. 006” • Polyester Patch • Short connecting waist • Sizes: 18, 25 and 35 mm

Endothelialization – Six Weeks Post-implant 35

Follow-up Schedule Pre-discharge (device only): • One month, six months, 12 months, 18 months, and 24 months, and annually until study end Six-month Visit: • TEE with bubble study required for device subjects 36

To Close or Not to Close: Summary • Closure of Patent Foramen Ovale (PFO) for cryptogenic stroke is controversial • Medical therapy carries low risk of complications • Device closure may carry a low peri-procedural risk compared to surgery • No randomized data published • Clinical trials are ongoing to evaluate device based closure and gain FDA approval for this indication

Management of Patients with PFO After Cerebral Events Therapy Recommended by PFO closure in patients with recurrent cryptogenic stroke despite medical therapy AAN Antiplatelet therapy Warfarin in the presence of deep vein thrombosis Warfarin in the presence of coagulation abnormalities Warfarin rather than antiplatelet agents in the presence of ASA AAN, AHA/ASA, ESO, ACCP AHA/ASA AAN ACCP= American College of Chest Physicians; AAN=American Association of Neurology; AHA/ASA=American Heart Association/American Stroke Association; and ESO, European Stroke Organization.

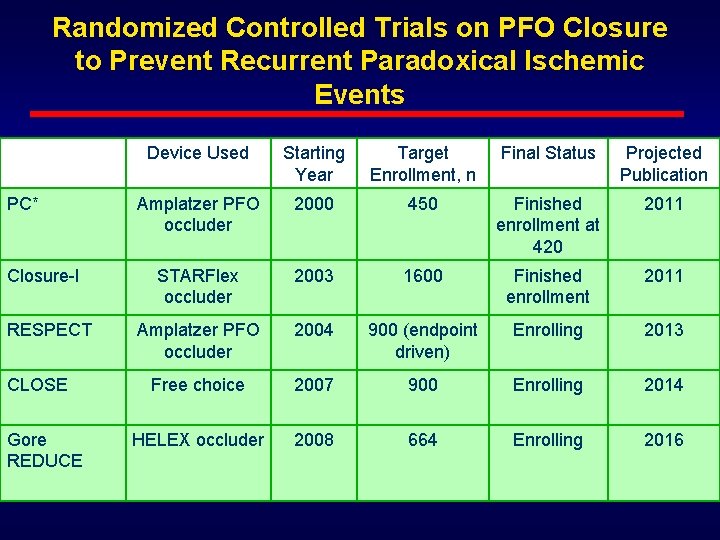
Randomized Controlled Trials on PFO Closure to Prevent Recurrent Paradoxical Ischemic Events PC* Closure-I RESPECT CLOSE Gore REDUCE Device Used Starting Year Target Enrollment, n Final Status Projected Publication Amplatzer PFO occluder 2000 450 Finished enrollment at 420 2011 STARFlex occluder 2003 1600 Finished enrollment 2011 Amplatzer PFO occluder 2004 900 (endpoint driven) Enrolling 2013 Free choice 2007 900 Enrolling 2014 HELEX occluder 2008 664 Enrolling 2016

Randomized Controlled Trials for PFO Closure in Migraine Headache Patients Acronym Place Sham Procedure Device Status UK + STARFlex Reported Global - Amplatzer Recruiting PREMIUM US + Amplatzer Recruiting MIST II US + Bio. STAR* Abandoned ESCAPE US + Premere† Abandoned Global + Flat. Stent‡ Planned MIST PRIMA ?

Top Ten Things to Know Percutaneous Device Closure of Patent Foramen Ovale for Secondary Stroke Prevention q Stroke is the third leading cause of death among adults in the United States and a major contributor to long-term functional impairment and disability. q The majority of strokes are ischemic; of these, about 2540% do not have identifiable cause after thorough evaluation and are designated as cryptogenic (CS). q A patent foramen ovale (PFO) is a remnant of the fetal circulation and has been identified at autopsy in 27% of patients with normal hearts.

Top Ten Things to Know Percutaneous Device Closure of Patent Foramen Ovale for Secondary Stroke Prevention q Many observational studies suggest a strong association between PFO and CS. But a causal relationship has not been convincingly established for most affected patients. q The optimal therapy for prevention of recurrent stroke or transient ischemic attack in patients with CS and PFO has not been defined. q Estimates of annual rates of recurrent stroke among patients with PFO range from 1. 5 – 12% and depend on the characteristics of the population studied, including age.

Top Ten Things to Know Percutaneious Device Closure of Patent Foramen Ovale for Secondary Stroke Prevention: 2010 q No device for PFO closure after cryptogenic stroke has been approved by the FDA. q Randomized controlled trials offer the best means for assessing the safety and efficacy of percutaneous device closure relative to anti-thrombotic meds. q Enrollment in ongoing clinical trials has lagged considerably despite frequent calls for participation from the FDA and major professional societies. q All cardiovascular clinicians should consider referral of patients with cryptogenic stroke and PFO an ongoing studies.