Petroleum Organic Chemistry Organic Chemistry Used to be
Petroleum Organic Chemistry
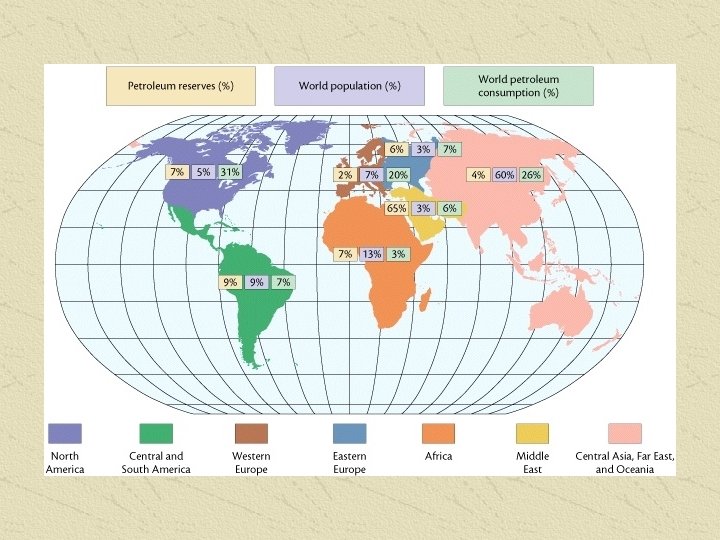

• Organic Chemistry Used to be considered chemistry of living things (or things that were once living… like petroleum) • Since it has been demonstrated that organic compounds can be synthesized in laboratories we now just say that Organic chemistry is the Chemistry of Hydrocarbon compounds • Examples: petroleum, medicines, plastics, plants and animals…. . . (including YOU!!)
• More than 90% of all known compounds contain Carbon (although it accounts for only 0. 2% of the earth’s crust composition) • Over 6, 000 organic compounds have been identified – and that number is increasing daily with the synthesis of new compounds in labs

• Hydrocarbons : simplest organic compounds containing only C and H atoms with “Carbon backbones” that are inherent to organic compounds

HOW DO YOU KNOW HOW C’s and H’s WILL GET TOGETHER TO FORM HYDROCARBONS? • The Octet Rule says that everybody (except H and He) wants to have 8 outer shell electrons, Carbon has only 4 (it’s glass is both half empty and half full) – so instead of giving or taking electrons (Ionic bonding) it SHARES electrons – A. K. A Covalent Bonding forms Molecular compounds – (organic stuff!)
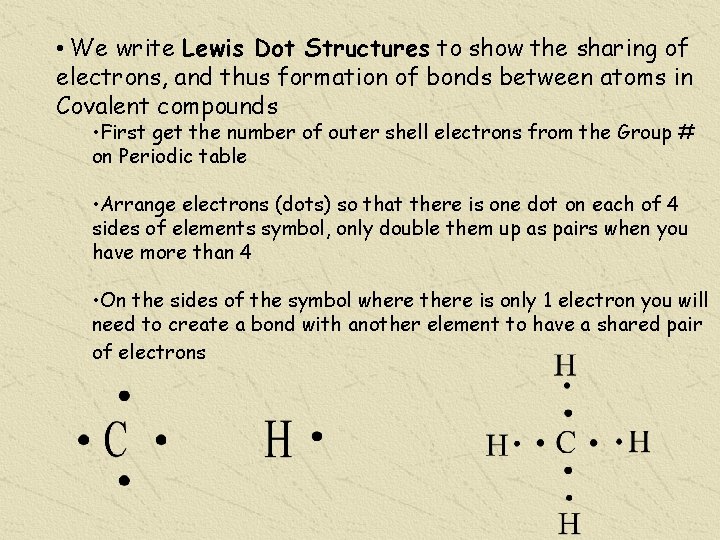
• We write Lewis Dot Structures to show the sharing of electrons, and thus formation of bonds between atoms in Covalent compounds • First get the number of outer shell electrons from the Group # on Periodic table • Arrange electrons (dots) so that there is one dot on each of 4 sides of elements symbol, only double them up as pairs when you have more than 4 • On the sides of the symbol where there is only 1 electron you will need to create a bond with another element to have a shared pair of electrons

• When Carbon shares electrons to fulfill it’s outer shell – it needs to share with other atoms to get 4 more electrons – so it needs to make 4 bonds • Hydrogen needs to make 1 bond • Oxygen needs to make 2 bonds • Nitrogen needs to make 3 bonds • Carbon needs to make 4 bonds
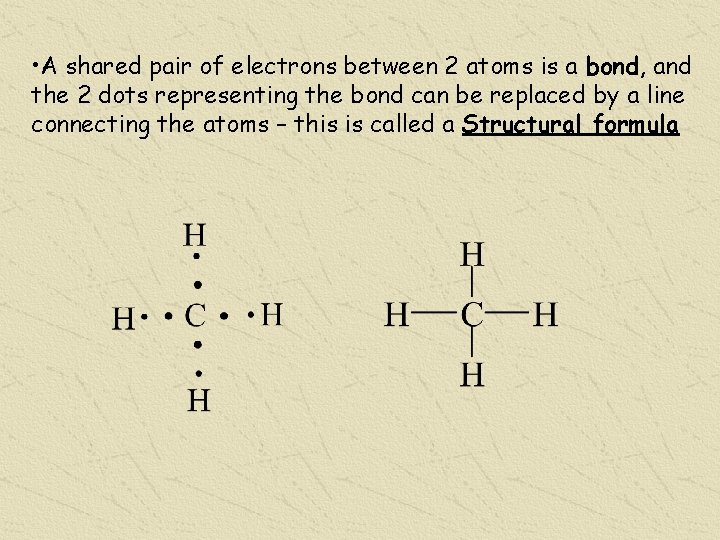
• A shared pair of electrons between 2 atoms is a bond, and the 2 dots representing the bond can be replaced by a line connecting the atoms – this is called a Structural formula
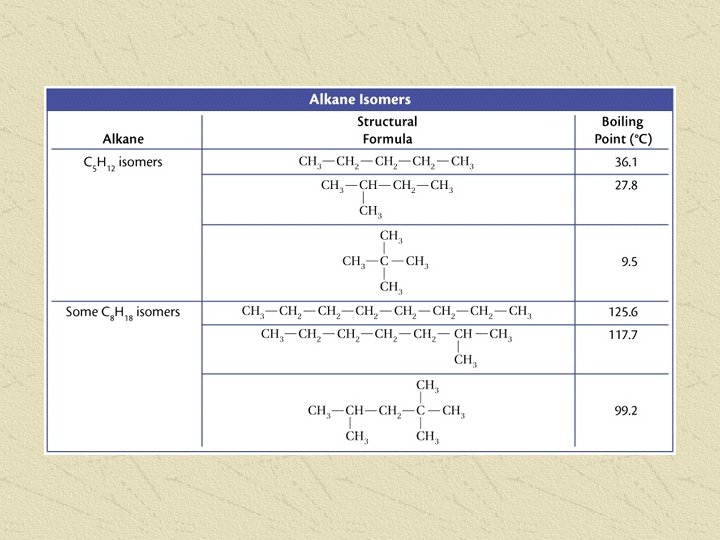
• Can also write MOLECULAR FORMULAS that do not show the individual bonds: • Expanded Molecular Formula still shows how atoms are arranged: • 2, propanol = CH 3 CH(OH)CH 3 Condensed Molecular Formula just shows the number of each atom: • 2, propanol = C 3 H 8 O Molecular modeling
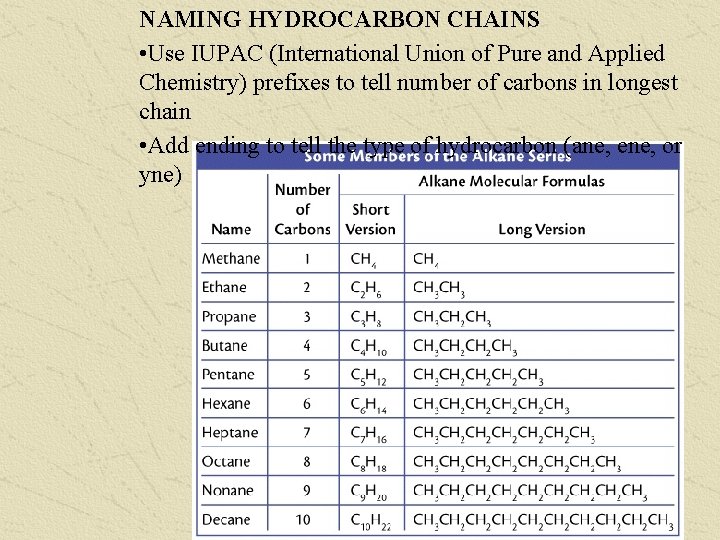
NAMING HYDROCARBON CHAINS • Use IUPAC (International Union of Pure and Applied Chemistry) prefixes to tell number of carbons in longest chain • Add ending to tell the type of hydrocarbon (ane, ene, or yne)
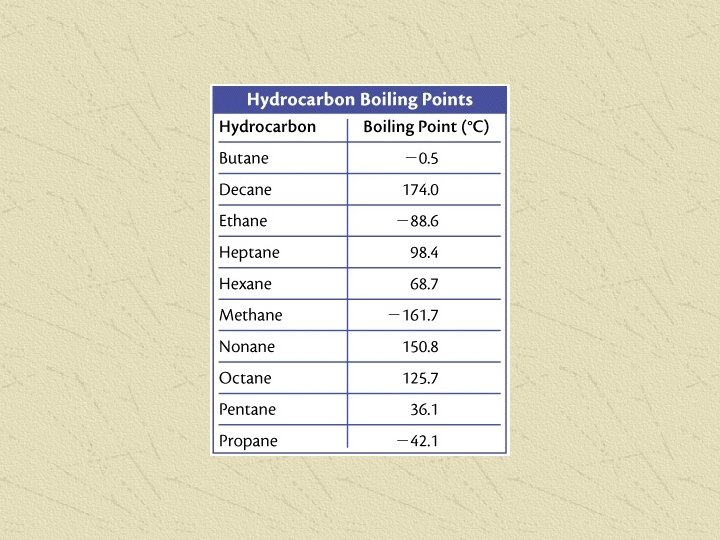
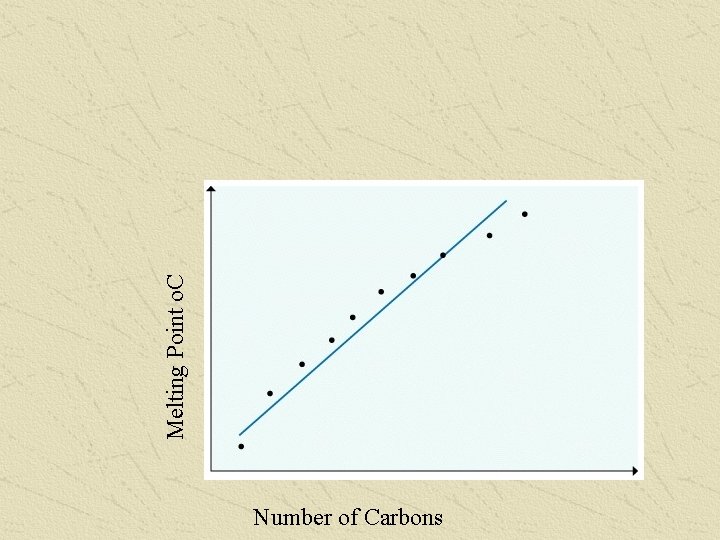
Melting Point o. C Number of Carbons
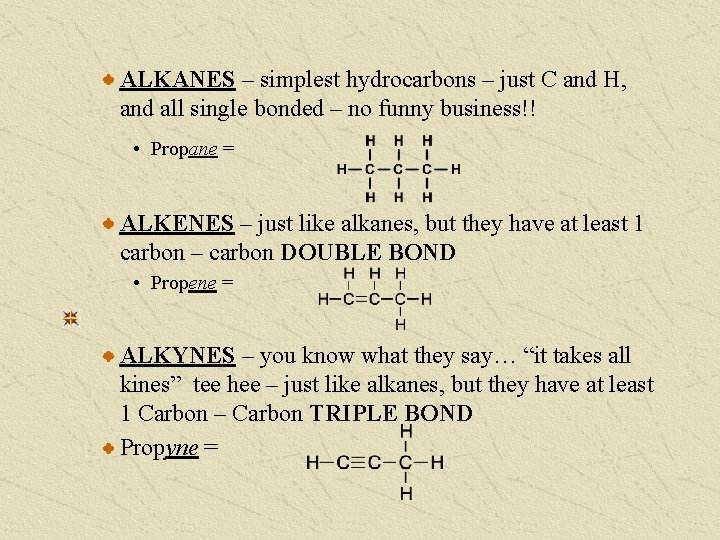
ALKANES – simplest hydrocarbons – just C and H, and all single bonded – no funny business!! • Propane = ALKENES – just like alkanes, but they have at least 1 carbon – carbon DOUBLE BOND • Propene = ALKYNES – you know what they say… “it takes all kines” tee hee – just like alkanes, but they have at least 1 Carbon – Carbon TRIPLE BOND Propyne =

ENERGY IN REACTIONS

ENERGY CONVERSION How many different types of energy can you think of? POTENTIAL – Stored Energy KINETIC – Energy in use (motion)
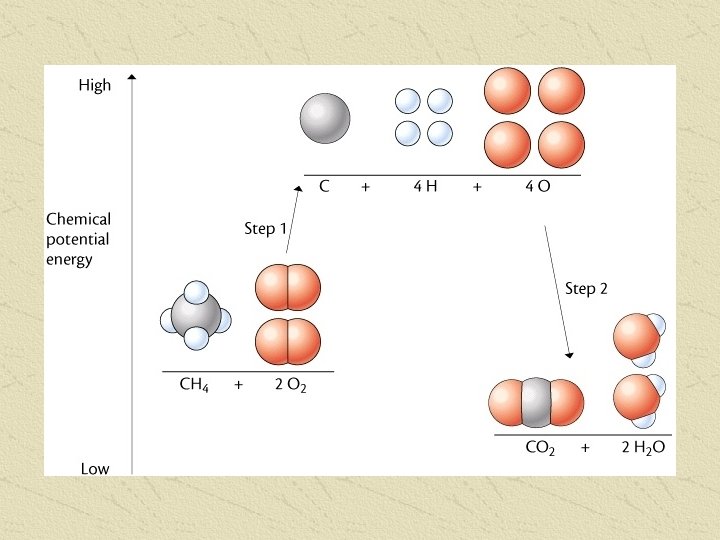
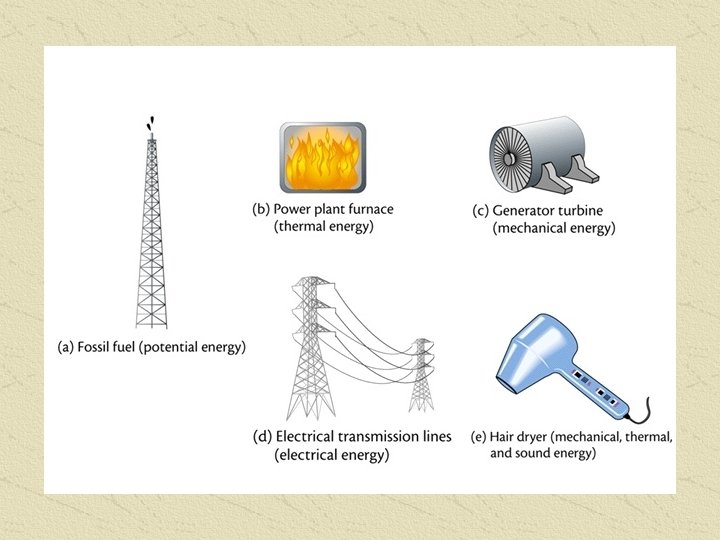
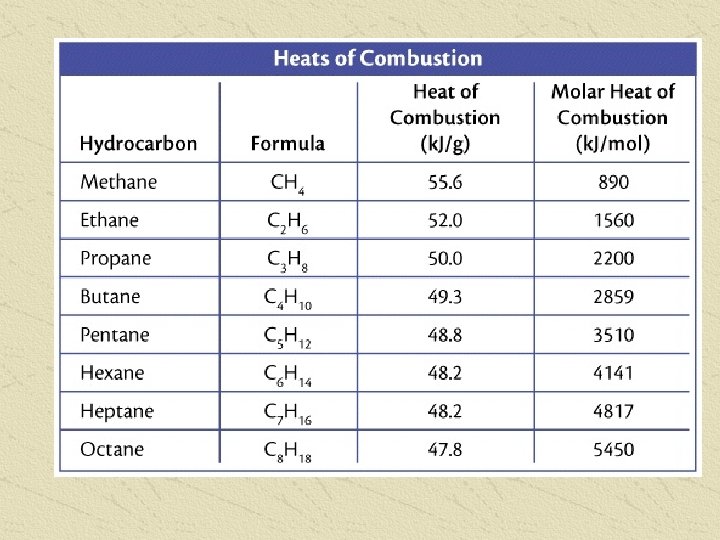
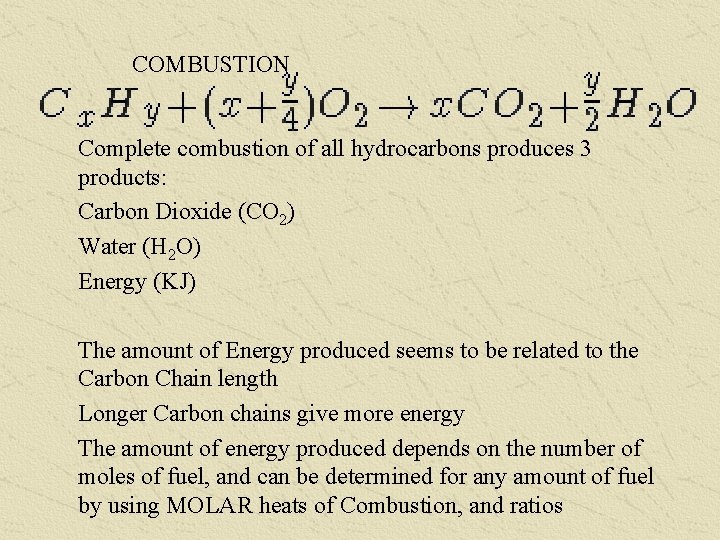
COMBUSTION Complete combustion of all hydrocarbons produces 3 products: Carbon Dioxide (CO 2) Water (H 2 O) Energy (KJ) The amount of Energy produced seems to be related to the Carbon Chain length Longer Carbon chains give more energy The amount of energy produced depends on the number of moles of fuel, and can be determined for any amount of fuel by using MOLAR heats of Combustion, and ratios
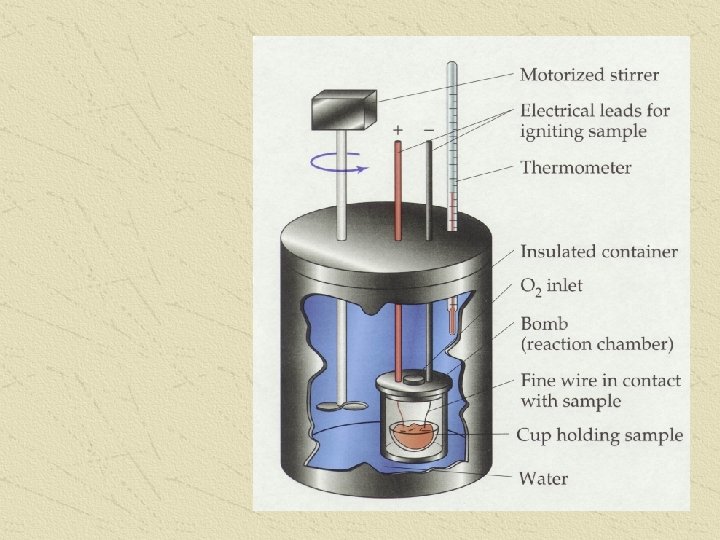
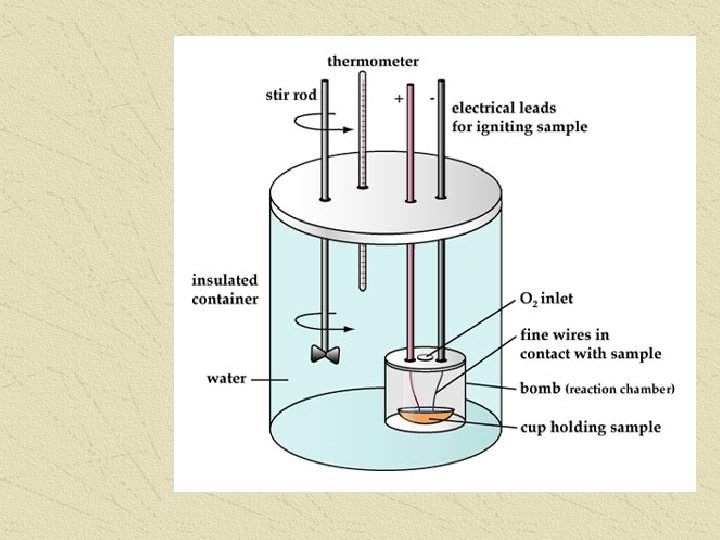
CALORIMETER or BOMB CALORIMETER
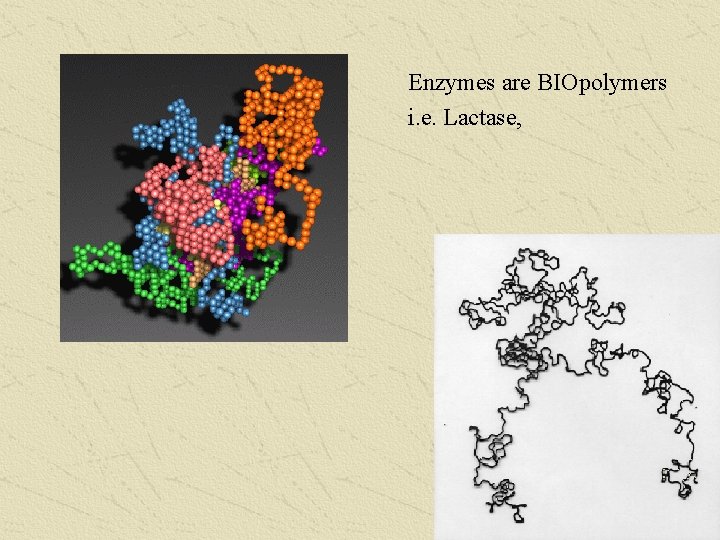
Enzymes are BIOpolymers i. e. Lactase,

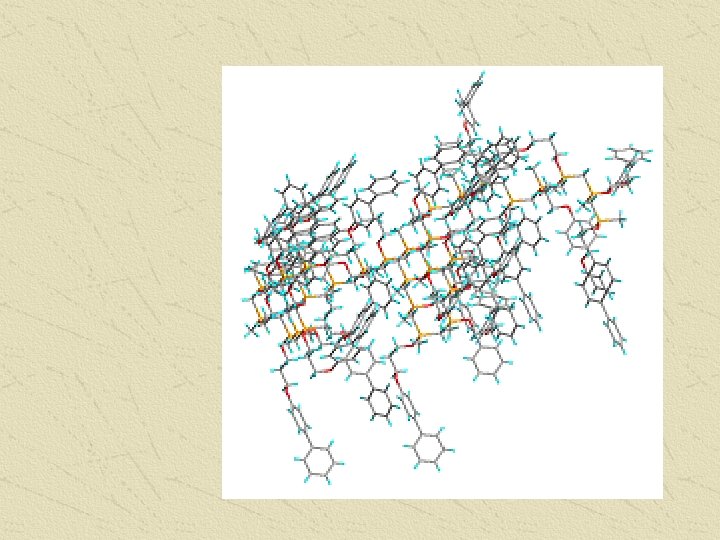
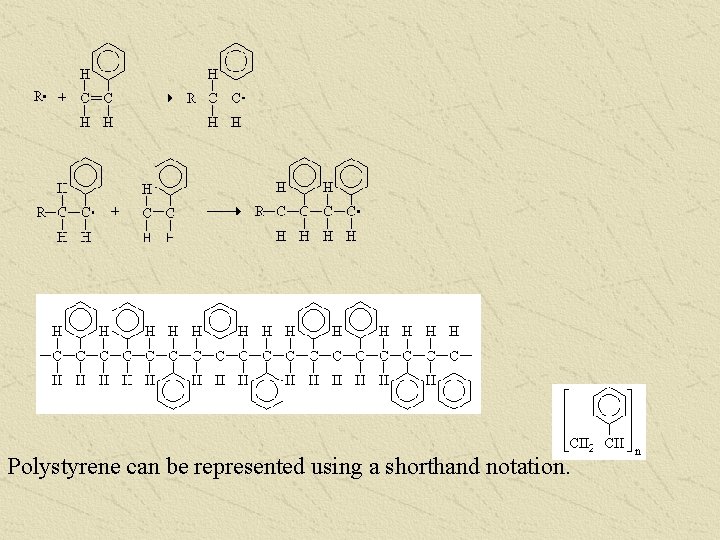
Polystyrene can be represented using a shorthand notation.
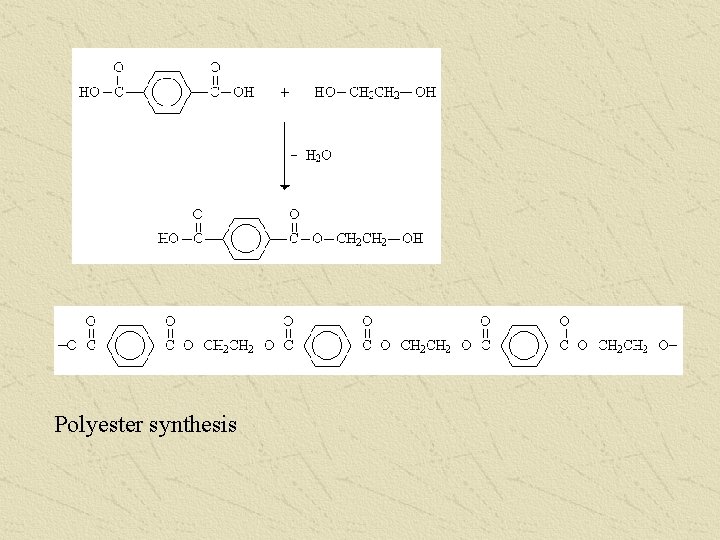
Polyester synthesis
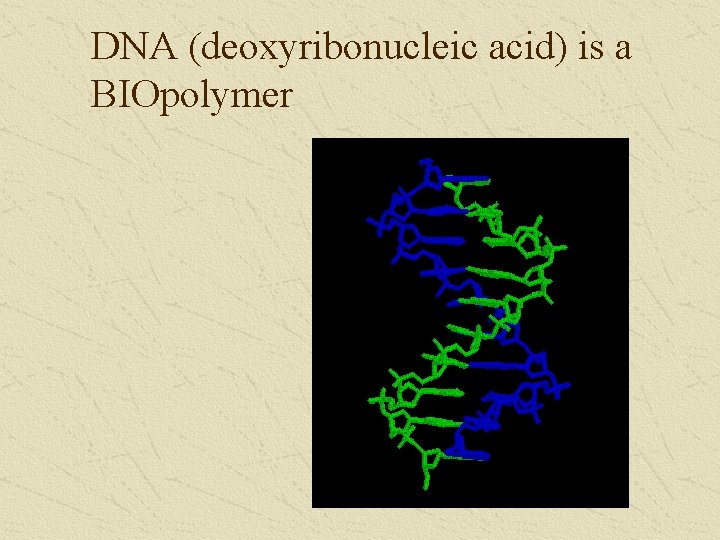
DNA (deoxyribonucleic acid) is a BIOpolymer
- Slides: 34