Peter Jackson Identifying Anions Nonmetal Ions Negative Ions












- Slides: 15

Peter Jackson Identifying Anions Non-metal Ions Negative Ions

Peter Jackson Introduction Peter Jackson • Anions are negative ions. • Called anions because they are attracted to the anode [positive electrode] during electrolysis. • Unlike charges attract • Some are simple ions e. g. Cl-, I-, Br-, S 2 -, N 3 • Others are complex ions or radicals e. g. SO 42 -, NO 3 -, CO 32 -, HCO 3 -,

Peter Jackson Introduction continued • Very often asked to distinguish between a pair of two similar anions. • SO 42 - / SO 32 • CO 32 - / HCO 31 -

Peter Jackson Basic Procedure • • What do you add? What do you see happening? Equation. [if required] What does this tell you? Sometimes • What else do you add? • What do you see happening? • What does this tell you?

Peter Jackson Chloride Cl • Place a little of the suspected chloride in a test tube and add some water. • Add some acidified silver nitrate solution (Silver nitrate plus nitric acid) • If the substance is a chloride then a white precipitate of silver chloride will form.

Peter Jackson • (This precipitate will turn purple and then black in sunlight) Ag. NO 3 (aq)+Na. Cl(aq) =Ag. Cl(s)+Na. NO 3(aq) Ag+ (aq) + Cl-(aq) = Ag. Cl (s) • Add some dilute ammonia solution [NH 4 OH] • The precipitate should re-dissolve

Peter Jackson Sulphate SO 42 • Place a little of the suspected sulphate in a test tube and add some water • Add some barium chloride solution. • A white precipitate of Barium sulphate will form if it is a sulphate Na 2 SO 4 (aq)+ Ba. Cl 2 (aq) = Ba. SO 4 (s) + 2 Na. Cl (aq) • Add some hydrochloric acid [HCl (aq)] to the precipitate it will not re-dissolve

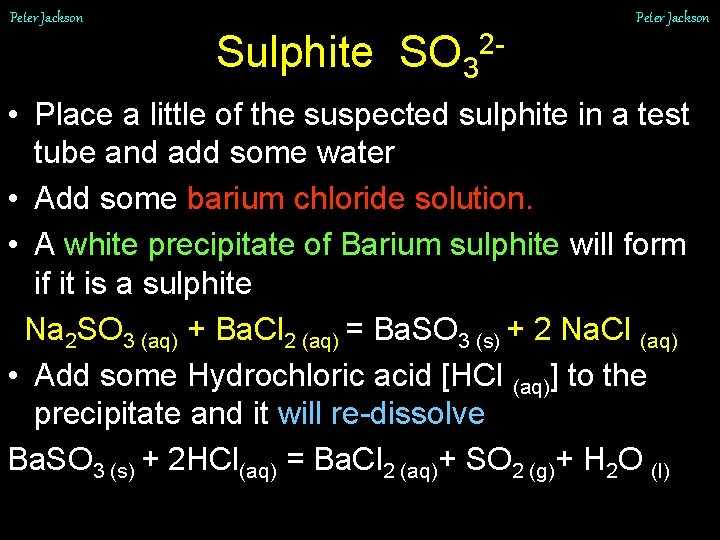
Peter Jackson Sulphite SO 32 • Place a little of the suspected sulphite in a test tube and add some water • Add some barium chloride solution. • A white precipitate of Barium sulphite will form if it is a sulphite Na 2 SO 3 (aq) + Ba. Cl 2 (aq) = Ba. SO 3 (s) + 2 Na. Cl (aq) • Add some Hydrochloric acid [HCl (aq)] to the precipitate and it will re-dissolve Ba. SO 3 (s) + 2 HCl(aq) = Ba. Cl 2 (aq)+ SO 2 (g)+ H 2 O (l)

Peter Jackson Carbonate CO 32 Hydrogencarbonate HCO 31 - Peter Jackson • Place a little of the suspected Carbonate in a test tube and add some dilute HCl • gas is produced test it with lime water. • If lime water turns milky then gas is CO 2 • This tells us that the substance is either a carbonate or a hydrogencarbonate • [If insoluble in water it is a carbonate. ]

Peter Jackson Carbonate CO 3 Hydrogencarbonate HCO 312 - Peter Jackson • If soluble take a fresh sample dissolve it in water and add magnesium sulphate solution. • If a white precipitate forms then it is a carbonate • If no precipitate forms it is a hydrogencarbonate

Peter Jackson Carbonate CO 3 • • • 2 - Place some carbonate in a test tube. Add dilute hydrochloric acid Effervescence occurs [fizzing] CO 32 -(s)+2 H+(aq)= CO 2(g)+ H 2 O(l) Test gas with lime water - turns milky so CO 2 produced. • Ca(OH)2(aq) + CO 2(g) = Ca. CO 3(s) + H 2 O(l) • Add Mg. SO 4 solution - white pptte forms • Mg 2+(aq) + CO 32 -(aq) = Mg. CO 3(s)

Peter Jackson Hydrogencarbonate HCO 3 • Place some hydrogencarbonate in a test tube. • Add dilute hydrochloric acid • Effervescence occurs [fizzing] • HCO 3 -(s)+ H+(aq)= CO 2(g)+ H 2 O(l) • Test gas with lime water - turns milky so CO 2 produced. • Ca(OH)2(aq) + CO 2(g) = Ca. CO 3(s) + H 2 O(l) • Add Mg. SO 4 solution - no pptte forms

Peter Jackson Nitrate NO 3 - • Brown Ring Test • Take a sample of the suspected nitrate and place it in a test tube • Dissolve it in water • if it does not dissolve it is not a nitrate because all nitrates are soluble in water • Add some iron(II)sulphate solution

Peter Jackson • Hold the test tube at 45 o and gently pour some concentrated sulphuric acid down the side • The sulphuric acid is more dense than the water and so will sink to the bottom forming two layers. • If the substance is a nitrate a brown ring will form between the two layers.

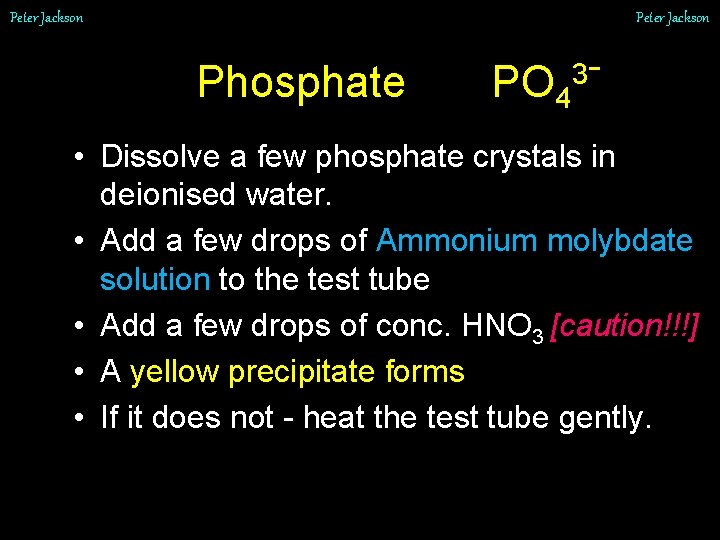
Peter Jackson Phosphate PO 4 3 - • Dissolve a few phosphate crystals in deionised water. • Add a few drops of Ammonium molybdate solution to the test tube • Add a few drops of conc. HNO 3 [caution!!!] • A yellow precipitate forms • If it does not - heat the test tube gently.