Personalized Cancer Medicine Winning the War on Cancer
























- Slides: 37

Personalized Cancer Medicine – Winning the War on Cancer Zhiyong Li, MD, Ph. D. Dallas Cancer Specialists 315 N. Shiloh Road, Suite 101 Garland, Texas 75042 972 -487 -8866

Personalized Cancer Medicine • • Cancer Burden Resource for fighting war on cancer Achievements Future of cancer medicine

Global Cancer Burden in 2012 • All sites • Breast New cases 14. 1 million 1. 7 million Cancer deaths 8. 2 million 522, 000 3

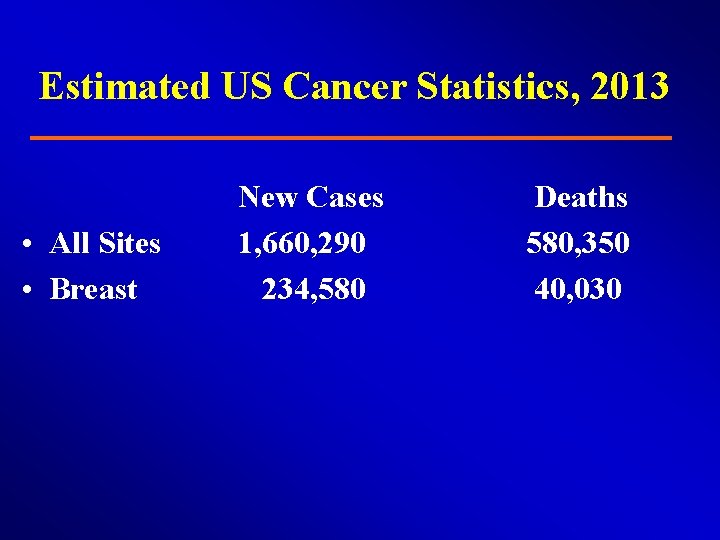
Estimated US Cancer Statistics, 2013 • All Sites • Breast New Cases 1, 660, 290 234, 580 Deaths 580, 350 40, 030

Bills for Cancer Research 1. The National Cancer Act - Enacted December 23, 1971 2. National Cancer Amendments - Enacted July 23, 1974 3. Biomedical Research and Research Training Amendments - Enacted November 9, 1978 4. Health Research Extension Act - Enacted November 20, 1985 5. Health Omnibus Program Extension - Enacted November 4, 1988 6. National Institutes of Health Reform – Enacted January 15, 2007

Resource spent for cancer research & treatment • NCI has spent some $90 billion • Some 260 nonprofit organizations in US have dedicated themselves to cancer (more than the number established for heart disease, AIDS, Alzheimer’s disease, and stroke combined). • These 260 organizations spent > $2. 2 billion annually • The United States has spent > a trillion dollars for war on cancer

Signal transduction pathways

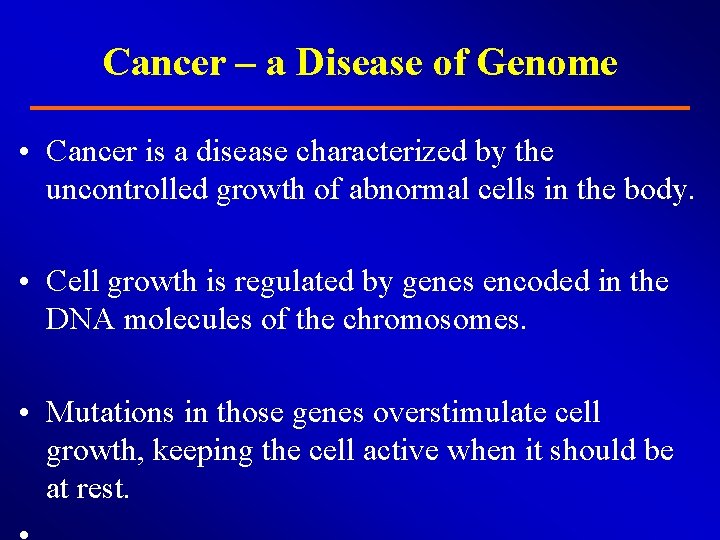
Cancer – a Disease of Genome • Cancer is a disease characterized by the uncontrolled growth of abnormal cells in the body. • Cell growth is regulated by genes encoded in the DNA molecules of the chromosomes. • Mutations in those genes overstimulate cell growth, keeping the cell active when it should be at rest.

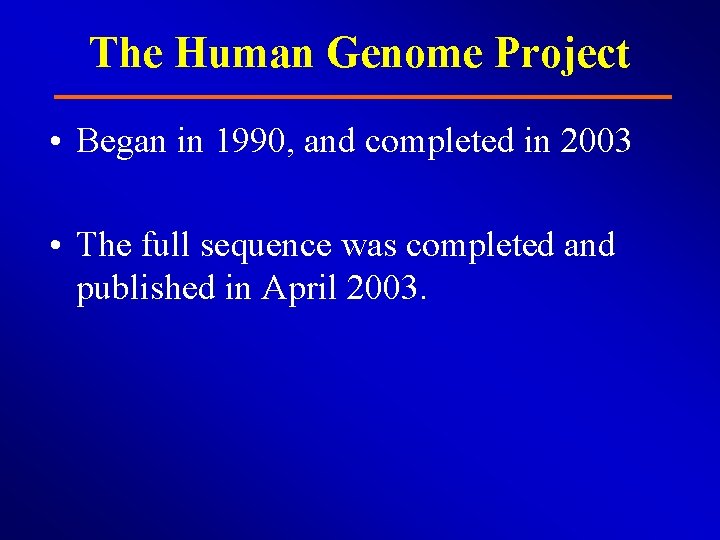
The Human Genome Project • Began in 1990, and completed in 2003 • The full sequence was completed and published in April 2003.

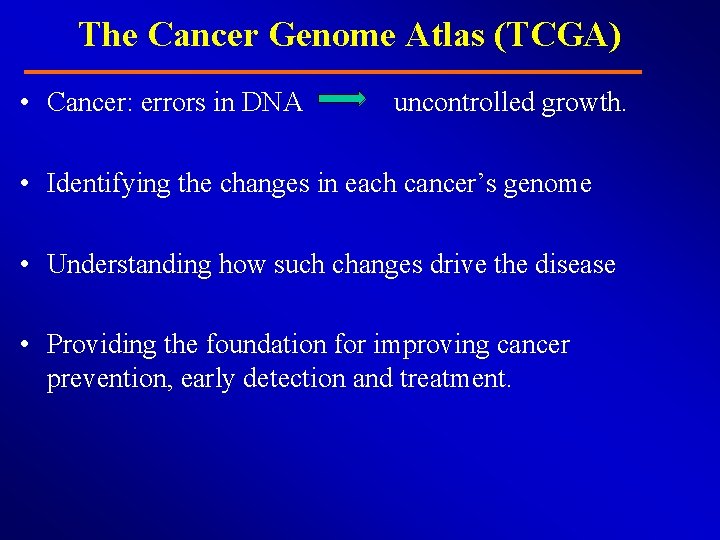
The Cancer Genome Atlas (TCGA) • Cancer: errors in DNA uncontrolled growth. • Identifying the changes in each cancer’s genome • Understanding how such changes drive the disease • Providing the foundation for improving cancer prevention, early detection and treatment.

The Cancer Genome Atlas (TCGA) • 2006 - TCGA began as a three-year pilot • National Cancer Institute and National Human Genome Research Institute committed $100 million • 2008 - TCGA reported first result: GBM • 2009 - began to map 20 cancers. • 2011 - 5, 000 th case ships to TCGA centers

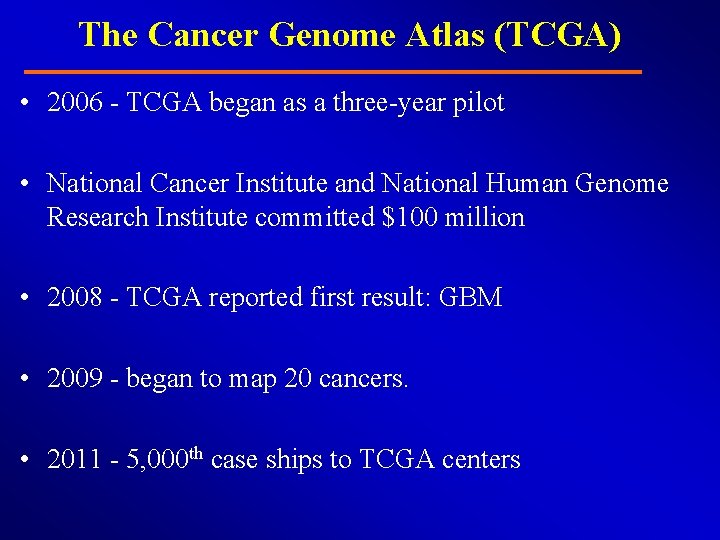
Her 2 pathway

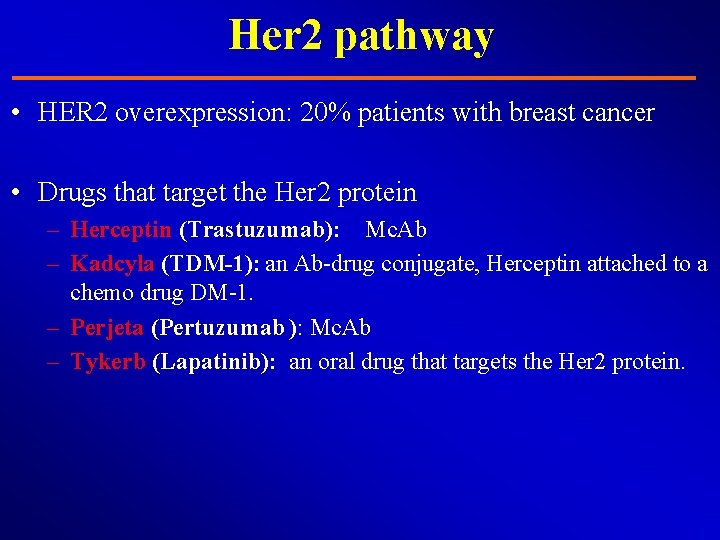
Her 2 pathway • HER 2 overexpression: 20% patients with breast cancer • Drugs that target the Her 2 protein – Herceptin (Trastuzumab): Mc. Ab – Kadcyla (TDM-1): an Ab-drug conjugate, Herceptin attached to a chemo drug DM-1. – Perjeta (Pertuzumab ): Mc. Ab – Tykerb (Lapatinib): an oral drug that targets the Her 2 protein.

PI 3 K/Akt/m. TOR pathway Growth factor receptor

PI 3 K/Akt/m. TOR pathway • the most frequently dysregulated in cancer. • Implicated in oncogenesis, progression, and resistance to conventional anticancer therapies. • Inhibition of this pathway has been shown to halt tumor growth, leading to tumor regression. • PI 3 K inhibitors showed synergistic activity with cytotoxic and targeted agents, and have restored sensitivity to these drugs •

PI 3 K/Akt/m. TOR pathway • • • Afinitor: approved for RCC and ER+ BC Torisel: approved for RCC Buparlisib (BKM 120): an oral pan-PI 3 K inhibitor. BYL 719 : PI 3 K a inhibitor XL 147: a selective PI 3 K inhibitor, XL 765: a dual m. TOR and PI 3 K inhibitor Idelalisib: PI 3 K d inhibitor, ? approval for CLL Pictilisib (GDC-0941): PI 3 K a/d inhibitor IPI – 145: PI 3 K d/g inhibitor

Cyclin-Dependent Kinase 4 and 6 (CDK 4/6) Pathway • Palbociclib - "breakthrough therapy" designated by FDA in 2013. • The phase II PALOMA-2 trial: • The phase III PALOMA-3 trial: • LEE 011: the most selective CDK 4/6 inhibitor • LY 2835219: CDK 4/6 inhibitor

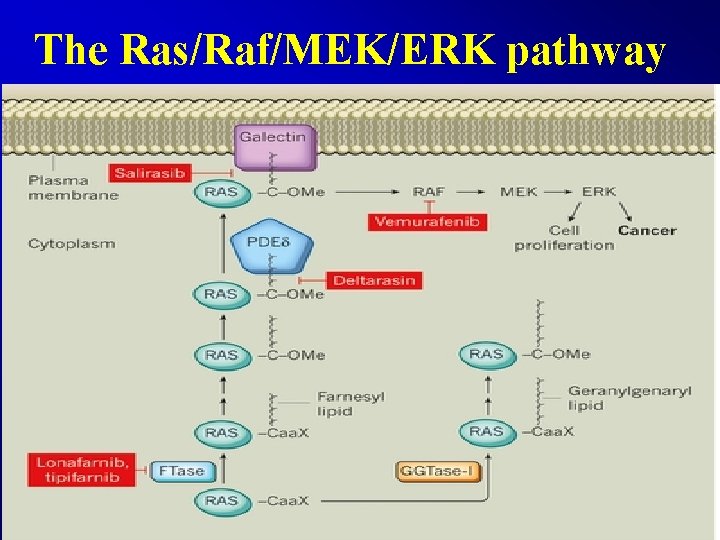
The Ras/Raf/MEK/ERK pathway

The Ras/Raf/MEK/ERK pathway • Vemurafenib, BRAF inhibitor, Melanoma • Dabrafenib, BRAF inhibitor, Melanoma • Trametinib, BRAF inhibitor, Melanoma • Dinaciclib • Lonafarnib and tipifarnib • Salirasib • Deltarasin

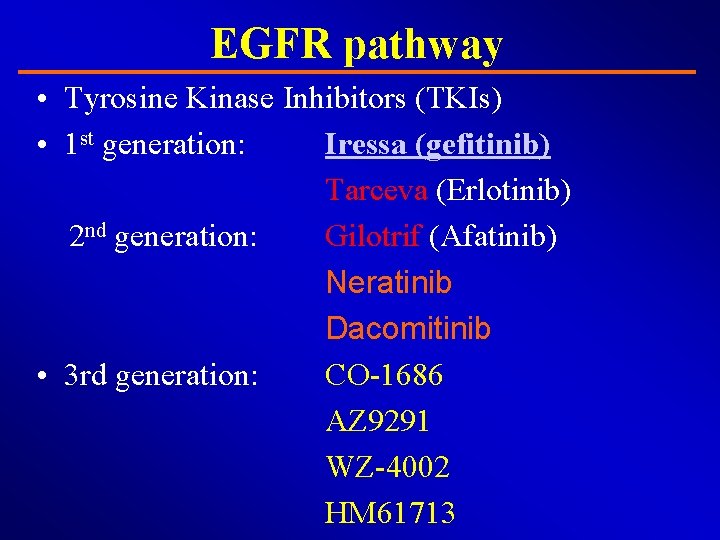
EGFR pathway • Tyrosine Kinase Inhibitors (TKIs) • 1 st generation: Iressa (gefitinib) Tarceva (Erlotinib) 2 nd generation: Gilotrif (Afatinib) Neratinib Dacomitinib • 3 rd generation: CO-1686 AZ 9291 WZ-4002 HM 61713

EGFR pathway • Monoclonal Antibody: Cetuximab (Erbitux) Vectibix (Panitumumab) Zalutumumab Nimotuzumab Matuzumab

ALK/ROS 1 pathway • Xalkori (Crizotinib) • Zykadia (Ceritinib), approved on 5/1/2014 • CH 5424802 • AP 26113

Foundation Medicine, Inc. • The company offers Foundation. One, a molecular information product for the analysis of routine cancer specimens, enabling physicians to provide targeted oncology therapies and optimize treatments.

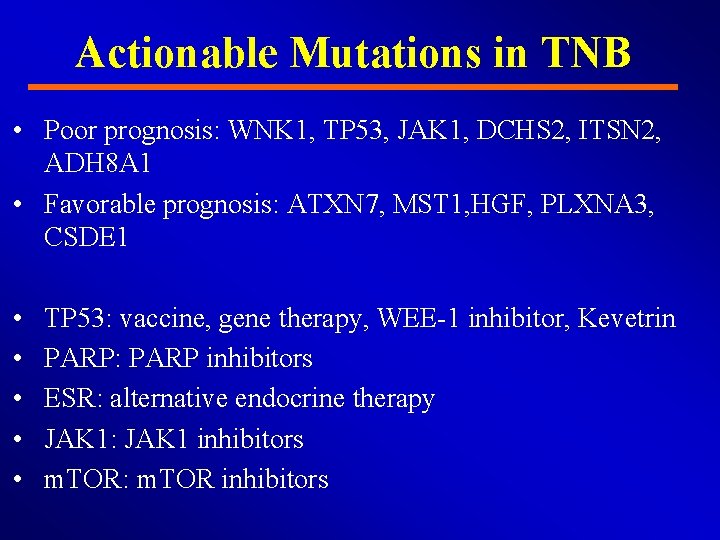
Actionable Mutations in TNB • Poor prognosis: WNK 1, TP 53, JAK 1, DCHS 2, ITSN 2, ADH 8 A 1 • Favorable prognosis: ATXN 7, MST 1, HGF, PLXNA 3, CSDE 1 • • • TP 53: vaccine, gene therapy, WEE-1 inhibitor, Kevetrin PARP: PARP inhibitors ESR: alternative endocrine therapy JAK 1: JAK 1 inhibitors m. TOR: m. TOR inhibitors

VEGF pathway • Avastin: a Mc. Ab targets VEGF. • Zaltrap: a fusion protein inhibits VEGF • VEGFR Tyrosine Kinase Inhibitors (TKIs) – Sutent (Sunitinib) – Votrient (Pazopanib) – Nexevar (Sorafenib) – Inlyta (Axitinib)

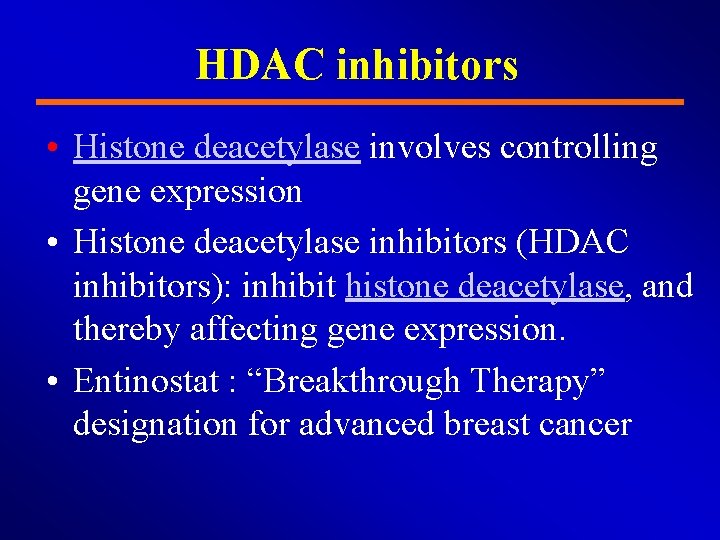
HDAC inhibitors • Histone deacetylase involves controlling gene expression • Histone deacetylase inhibitors (HDAC inhibitors): inhibit histone deacetylase, and thereby affecting gene expression. • Entinostat : “Breakthrough Therapy” designation for advanced breast cancer

Cancer Immunotherapy • Provenge (sipuleucel-T) Approved in April 29, 2010 A dendritic cell vaccine for m. CRPC

Cancer Immunotherapy • Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4) inhibitor – Functioning as a brake on T cells, – Preventing T cells from launching full-out immune attacks. Blocking the CTLA-4 molecule would set T cells free to destroy cancer. – In 2011, the U. S. FDA approved anti–CTLA-4 treatment, Yervoy (ipilimumab, BMS), for metastatic melanoma.

Cancer Immunotherapy • Programmed Death 1 (PD-1) inhibitor – another brake on T cells. • Blocking PD-1 by an anti–PD-1 antibody would set T cells free to destroy cancer. – Nivolumab (Bristol-Myers Squibb) – MK-3475 (Merck & Co) – MPDL 3280 A (Roche's Genentech) – MEDI 4736 (Aztra. Zeneca)

Cancer Immunotherapy • Chimeric Antigen Receptor therapy (CAR therapy) – A personalized treatment involves genetically modifying a patient's T cells to make them target tumor cells. – Highly effective in leukemia and lymphoma.

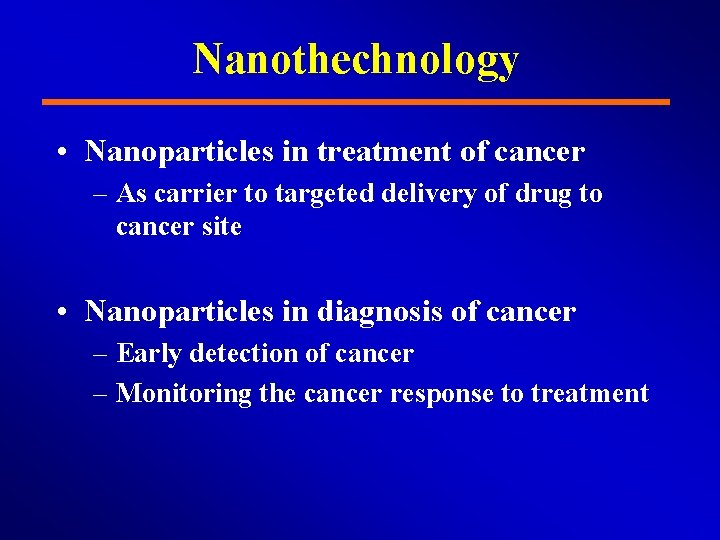
Nanothechnology • Nanoparticles in treatment of cancer – As carrier to targeted delivery of drug to cancer site • Nanoparticles in diagnosis of cancer – Early detection of cancer – Monitoring the cancer response to treatment

Successful Story • Chronic Myeloid Leukemia (CML) – Most fatal disease, median survival ~ 3 years. – Prior to 2001, only treatment: • Chemotherapy • Interferon • BMT

Successful Story • Chronic Myeloid Leukemia (CML) – Gleevec was 1 st TKI approved in 2001 – The most effective with minimal side effect. – Targeted therapy for BCR/ABL gene rearrangement – Now median survival for CML – projected 30 years. – New 2 nd and 3 rd generation • • • Tasigna Sprycel Bosutinib Ponatinib (Iclusig) Omacetaxine (Synribo).

Chronic Lymphocytic Leukemia • Median survival: 15 -20 years • Ibrutinib is 1 st BTK inhibitor approved for CLL in Feb, 2014 • Idelalisib is PI 3 K inhibitor waiting for approval for CLL

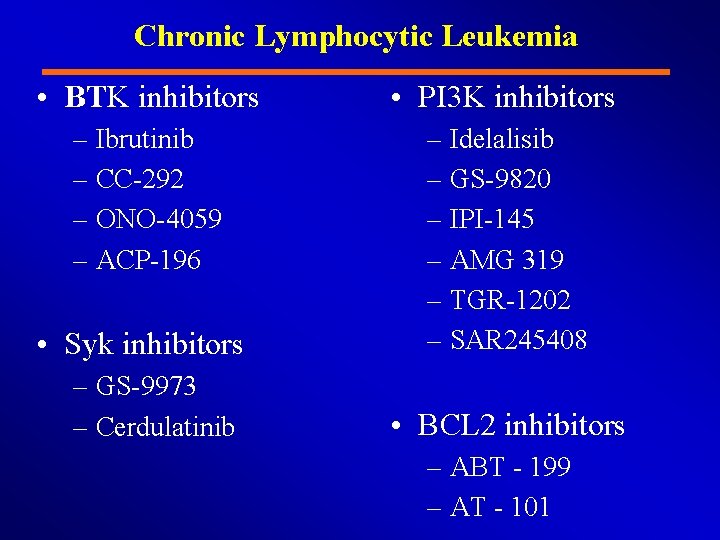
Chronic Lymphocytic Leukemia • BTK inhibitors • PI 3 K inhibitors – Ibrutinib – CC-292 – ONO-4059 – ACP-196 – Idelalisib – GS-9820 – IPI-145 – AMG 319 – TGR-1202 – SAR 245408 • Syk inhibitors – GS-9973 – Cerdulatinib • BCL 2 inhibitors – ABT - 199 – AT - 101

Personalized Cancer Medicine Will Win the War on Cancer

Cancer Awareness Program for Patient Advocate & Public Education for Public Dallas Cancer Specialists 315 N. Shiloh Road, Suite 101 Garland, Texas 75042 972 -487 -8866
 Mdanderson.org
Mdanderson.org Winning the war chapter 4 section 4
Winning the war chapter 4 section 4 Personalized social stories
Personalized social stories Personalized patient education
Personalized patient education First draft personal statement
First draft personal statement Adversarial personalized ranking for recommendation
Adversarial personalized ranking for recommendation Personalized learning learner profile
Personalized learning learner profile Contextual bandits for personalized recommendation
Contextual bandits for personalized recommendation Communication operations
Communication operations Personalized mobile search engine ieee paper
Personalized mobile search engine ieee paper Institute for personalized learning
Institute for personalized learning Oyunlarm
Oyunlarm Dietmar jannach
Dietmar jannach Limitations of trait theory of leadership
Limitations of trait theory of leadership Pentaho business analytics integrations
Pentaho business analytics integrations Personalized navigation
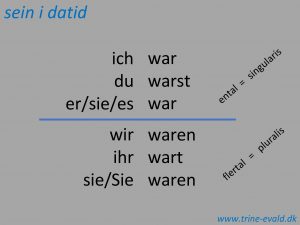
Personalized navigation Ich war du warst er war
Ich war du warst er war Description
Description Chapter 30 the war to end war
Chapter 30 the war to end war Ich war du warst
Ich war du warst Chapter 16 lesson 2 challenges to slavery
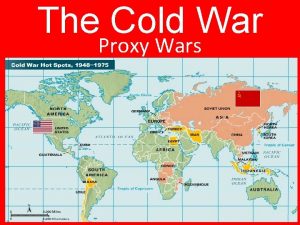
Chapter 16 lesson 2 challenges to slavery The cold war begins lesson 1
The cold war begins lesson 1 Contact force examples
Contact force examples Proxy wars cold war
Proxy wars cold war Comparing reconstruction plans venn diagram
Comparing reconstruction plans venn diagram Civil war first modern war
Civil war first modern war War at home vs war abroad madison
War at home vs war abroad madison Chapter 30 the war to end war
Chapter 30 the war to end war Why did josette dugas want to go to war
Why did josette dugas want to go to war Up student plagiarizes prize-winning photos
Up student plagiarizes prize-winning photos Core abilities
Core abilities Grasp teaching strategy
Grasp teaching strategy Winning executive summary
Winning executive summary Misere nim solution
Misere nim solution Winning at math paul nolting
Winning at math paul nolting Word limit of diary entry
Word limit of diary entry Evangelical church winning all
Evangelical church winning all How to design a winning business model
How to design a winning business model