Peritoneal Nodules and Cytoreductive Surgery By dr Ahmed


Peritoneal Nodules and Cytoreductive Surgery By dr. Ahmed Samir Surgical oncology senior registrar MD, Msc, MBBch

Etymology �“Peritoneum” is derived from the greek word peritonaion: peri means "around, " while teino means "to stretch"; thus, "peritoneum" means "stretched over.

Introduction �Peritoneal carcinomatosis (GI and ovarian), mesothelioma, and sarcomatosis are included in the group of diseases collectively referred to, as peritoneal metastases. �It is associated with short survival and poor quality of life, and may lead to bowel obstruction, accumulation of fluid in the peritoneal cavity and pain. NICE, 2009

�CRS with HIPEC has been used with variable success to treat pseudomyxoma peritonei, appendiceal mucinous neoplasias, peritoneal mesothelioma, PC from gastric, colorectal, and ovarian cancer, and other primary peritoneal surface malignancies. (Yan et al, 2009) (Chua et al, 2010) (Bakrin et al, 2012) (Alexander et al, 2013)

It is a loco regional disease but not a metastatic process, can be taken curative intent (Sugarbaker, 1989). �Ovarian �Gastric �Colorectal �Pancreatic
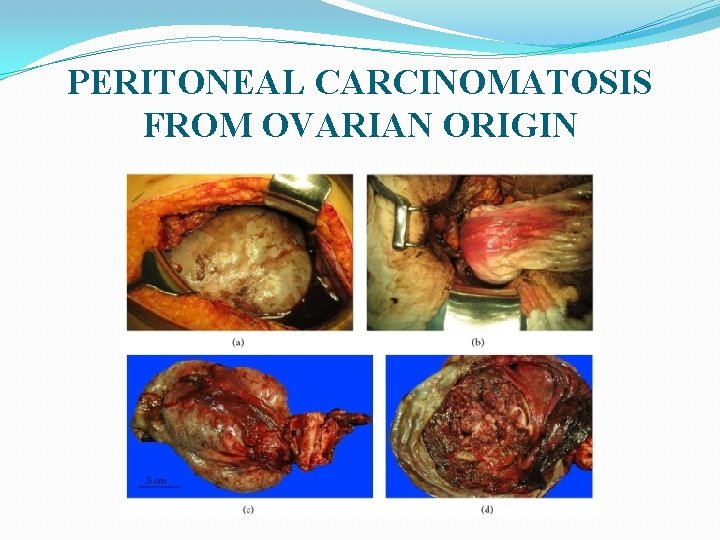
PERITONEAL CARCINOMATOSIS FROM OVARIAN ORIGIN

�Ovarian epithelial and primary peritoneal cancers are a major cause of mortality in developed countries �Ovarian and peritoneal carcinomas tend to be a peritoneal surface disease for a considerable part of their natural history, with systemic metastatic disease usually being observed late in the natural history of the disease. (Jemal et al, 2011).

� One of the most distinct features of Epithelial Ovarian Cancer is the tendency to disseminate into the peritoneal cavity and remain confined to the peritoneum and intraabdominal viscera. This makes it an ideal target for locoregional therapy. � HIPEC has become a useful therapeutic strategy to obtain a higher degree of debulking by trying to eliminate the residual microscopic component responsible for recurrences (Evgenia et al, 2015).
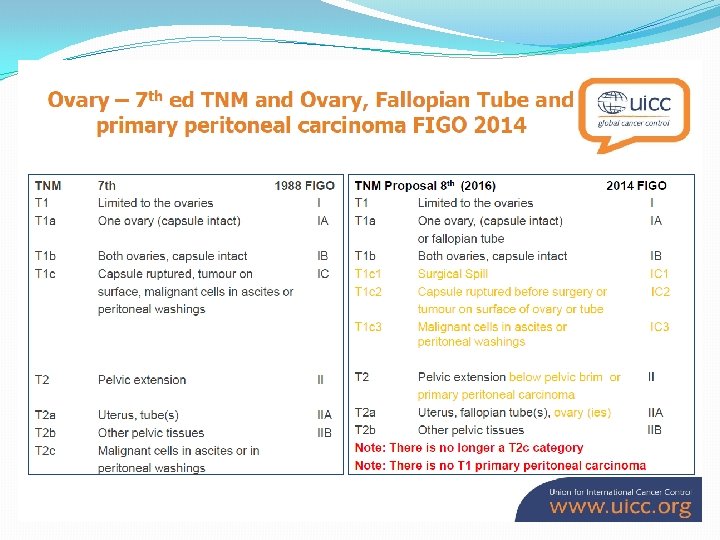
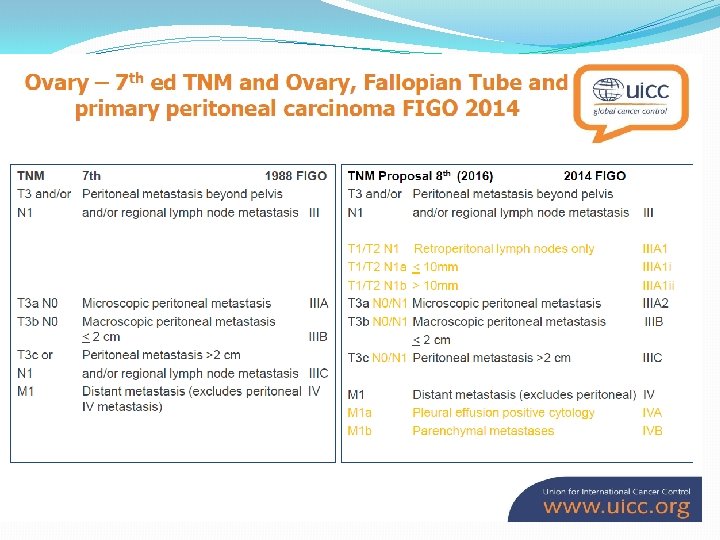

�A meta-analysis of 6, 885 patients undergoing maximal cytoreductive surgery (CRS) has shown a distinct survival advantage for those with maximal tumour removal and minimum residual disease (Bristow et al, 2002).

Peritonectomy procedures and resections that are combined to complete cytoreduction procedure (Evgenia et al, 2015).

PERITONEAL CARCINOMATOSIS FROM GASTRIC ORIGIN

�Gastric cancer (GC) is the second leading cause of cancer death and the fourth most common cancer in the world (Parkin et al, 1999) (Kelley et al, 2003). �The 40% of patients died for GC have hepatic metastases, while the 53 -60% showed a disease progression and died with peritoneal carcinosis (PC). The two most important factors affecting prognosis in GC are the serosal invasion and the lymphatic spread (Yu et al, 1995).
�PC is already present in 5 -20% of patients explored for potentially curative resection also in early gastric cancer (Kuramoto et al, 2009). �In contrast to lymphatic and haematogenous dissemination, peritoneal spread should be regarded as a locoregional disease extension rather than systemic metastasis (Yan et al, 2007).
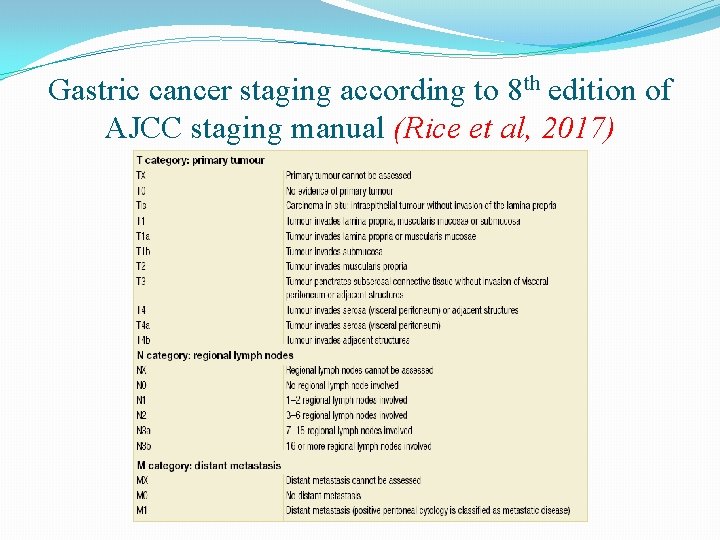
Gastric cancer staging according to 8 th edition of AJCC staging manual (Rice et al, 2017)
�A positive effect of IPC has been found on overall , peritoneal recurrence and on distant metastasis. Morbidity rate is incremented by IPC. Loco-regional lymph-nodes invasion in patients affected by advanced gastric cancer is not a contraindication to IPC (Coccolini et al, 2013).
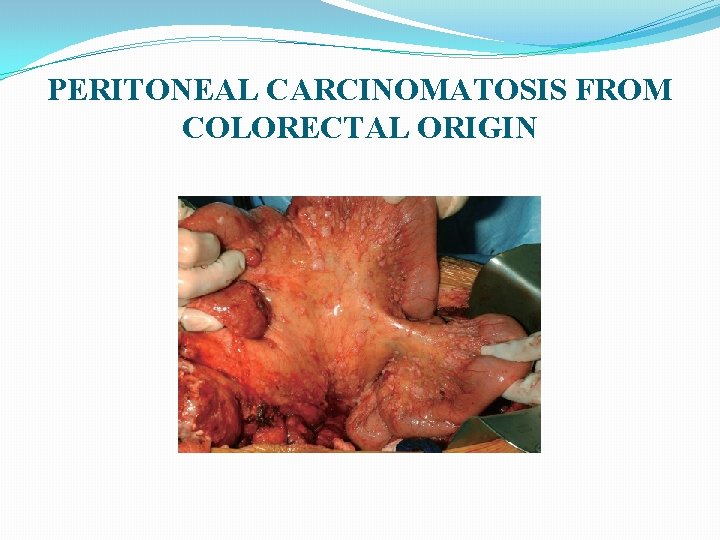
PERITONEAL CARCINOMATOSIS FROM COLORECTAL ORIGIN
�Globally, colorectal cancer (CRC) is the third leading cause of cancer, totaling 1. 6 million incident cases in 2013, and the fourth leading cause of cancer-related mortality, accounting for 771, 000 deaths (Fitzmaurice et al, 2015). �Peritoneal carcinomatosis (PC) is present in about 4– 15% of patients with CRC at initial diagnosis and in up to 50% in recurrent disease following curative resection (Segelman et al, 2012).
Pathophysiology of Peritoneal Carcinomatosis From GI Malignancies �PC is thought to be a locoregional disease with two main mechanisms that result in peritoneal spread of the primary tumor: (1) transmural tumor invasion that results in the exfoliation of free cells, which directly spread to the peritoneum; (2) visceral perforation or surgical trauma that causes cell spillage from the bowel lumen or the dissected vasculature that harbor tumor cells in transit (Stewart et al, 2005).

�Peritoneal spread results from a cascade of events that start by the loss of cell-cell adhesion molecules , followed by anoikis resistance, which is cell resistance to apoptosis and usually occurs when a normal cell loses cell matrix contact. Thereby, tumor cells migrate and adhere to the peritoneal surface through integrin and cadherin proteins. Then, using proteolytic enzymes such as matrix metallopeptidase, the tumor cells digest the extracellular matrix, facilitating invasion, colonization, and finally, homing to the peritoneum (de Cuba et al, 2012)(Schempp et al 2014).

Colorectal cancer staging according to 8 th edition of AJCC staging manual (Rice et al, 2017)
�A multidisciplinary approach. �patients with extraperitoneal disease or bulky retroperitoneal disease are not eligible for CRS with HIPEC. �helical CT scan with IV contrast (bulky peritoneal disease). �diffusion-weighted magnetic resonance imaging (DWMRI) (small peritoneal implants). (Ibrahim et al, 2017)

�Only patients with disease amenable to complete cytoreduction (R 0/1) should be considered since incomplete cytoreduction (R 2) is associated with worse survival.
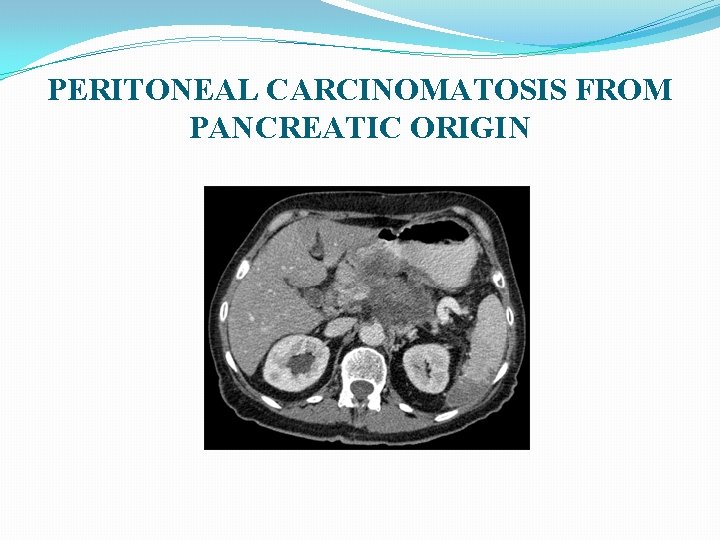
PERITONEAL CARCINOMATOSIS FROM PANCREATIC ORIGIN
�In comparison to other gastrointestinal malignancies, the surgery employed to date for pancreas cancer should be considered a failure as Long term survival following pancreaticoduodenectomy for adenocarcinoma is ten percent or less (winter et al, 2006). �The anatomic position of the pancreas deep in the retroperitoneal part of the upper abdomen causes “no touch cancer resection” to be impossible (Sugarbaker, 2017).
�In the process of performing the pancreaticoduodenectomy with clear margins, cancer cells may gain access to the peritoneal space and grow out at high density at the resection site. The phenomenon has been called tumor cell entrapment. �Peritoneal metastases occur prior to the pancreatic resection in an estimated 10% of patients. However, after the pancreatectomy in patients who had no peritoneal metastases at the time of resection, 50% or more patients will develop local recurrence and/ or peritoneal metastases in follow-up (Sugarbaker, 2017).

�Numerous trials and meta-analyses have attempted to establish a benefit for radiochemotherapy for pancreas cancer either before or after cancer resection. However, none of them have established this as a preferred method of treatment (Khorana et al, 2016).

�A profound effect of HIPEC gemcitabine used following pancreatectomy and prior to intestinal reconstruction on cancer cells lost into the peritoneal space during the cancer resection is suggested. �Reduced local recurrence and peritoneal metastases post operatively. �HIPEC gemcitabine shows promise to reduce peritoneal seeding in patients having pancreas cancer resection in the absence of increased morbidity and mortality. (Sugarbaker, 2017).

Refrences � A. A. Khorana, P. B. Mangu, J. Berlin, et al. , Potentially curative pancreatic cancer: american Society of Clinical Oncology clinical practice guideline, J. Clin. Oncol. 34 (2016) 2541 -2556. � Alexander HR, Bartlett DL, Pingpank JF, Libutti SK, Royal R, Hughes MS, et al. Treatment factors associated with long -term survival after cytoreductive surgery and regional chemotherapy for patients with malignant peritoneal mesothelioma. Surgery. 2013; 153: 779– 86. � Bakrin N, Cotte E, Golfier F, Gilly FN, Freyer G, Helm W, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for persistent and recurrent advanced ovarian carcinoma: a multicenter, prospective study of 246 patients. Ann Surg Oncol. 2012; 19: 4052– 8. Springer-Verlag. � Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002; 20: 1248 -1259. � Chua TC, Morris DL, Esquivel J. Impact of the peritoneal surface disease severity score on survival in patients with colorectal cancer peritoneal carcinomatosis undergoing complete cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2010; 17: 1330– 6. Springer-Verlag. � de Cuba EMV, Kwakman R, Van Egmond M, Bosch LJW, Bonjer HJ, Meijer GA, et al. Understanding molecular mechanisms in peritoneal dissemination of colorectal cancer. Virchows Arch. 2012; 461: 231– 43. Springer-Verlag. � Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, Mac. Intyre MF, et al. The global burden of cancer 2013. JAMA Oncol. 2015; 1: 505– 23. � Ibrahim Nassour & Patricio M. Polanco. Current Management of Peritoneal Carcinomatosis From Colorectal Cancer: the Role of Cytoreductive Surgery and Hyperthermic Peritoneal Chemoperfusion. Curr Colorectal Cancer Rep (2017) 13: 144– 153 � J. M. Winter, J. L. Cameron, K. A. Campbell, et al. , 1423 pancreaticoduodenectomies for pancreatic cancer: a single institution experience, J. Gastrointest. Surg. 10 (2006) 1199 -1210. � Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61: 69 -90. � Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol 2003; 56(1): 1– 9.

� � � � Kuramoto M, Shimada S, Ikeshima S, et al. Extensive intraoperative peritoneal lavage as a standard prophylactic strategy for peritoneal recurrence in patients with gastric carcinoma. Ann Surg 2009; 250: 242– 6. NICE. Interventional procedure overview of cytoreduction surgery followed by hyperthermic intraoperative peritoneal chemotherapy for peritoneal carcinomatosis. IPG 331. London: National Institute for Health and Clinical Excellence, 2009. Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin 1999; 49(1): 33– 64. Paul H. Sugarbaker. Strategies to improve local control of resected pancreas adenocarcinoma. Surgical Oncology 26 (2017) 63 -70 Rice TW, Kelsen DP, Blackstone EH, et al. gastric cancer. In: Amin MB, Edge SB, Greene FL, et al. , editors. AJCC Cancer Staging Manual, 8 th ed. New York: Springer, 2017: 185 -202. Schempp CM, von Schwarzenberg K, Schreiner L, Kubisch R, Müller R, Wagner E, et al. V-ATPase inhibition regulates anoikis resistance and metastasis of cancer cells. Mol Cancer Ther. 2014; 13: 926– 37. American Association for Cancer Research. Segelman J, Granath F, Holm. T, Machado M, Mahteme H, Martling A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2012; 99: 699– 705. John Wiley & Sons, Ltd. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Jaime Prat; for the FIGO Committee on Gynecologic Oncology. International Journal of Gynecology and Obstetrics 124 (2014) 1– 5 Stewart JH, Shen P, Levine EA. Intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: current status and future directions. Ann Surg Oncol. 2005; 12: 765– 77. Springer-Verlag. Sugarbaker PH, Cuncliffe WJ, Belliveau J, de Bruijn EA, Graves T, Mullins RE, et al. (1989) Rationale for integrating early postoperative intraperitoneal chemotherapy into the surgical treatment of gastrointestinal cancer. Semin Oncol; 16: 83 -97 Sugarbaker PH. Management of peritoneal carcinomatosis. Acta Med Austriaca. 1989; 16(3 -4): 57 -60. Yan TD, Black D, Sugarbaker PH, et al. A systematic review and meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for resectable gastric cancer. Ann Surg Oncol 2007; 14: 2702– 13. Yan TD, Deraco M, Baratti D, Kusamura S, Elias D, Glehen O, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multiinstitutional experience. J Clin Oncol. 2009; 27: 6237– 42. American Society of Clinical Oncology. Yu CC, Levison DA, Dunn JA, et al. Pathological prognostic factors in the second British Stomach Cancer Group trial of adjuvant therapy in resectable gastric cancer. Br J Cancer 1995; 71: 1106– 10.

- Slides: 33