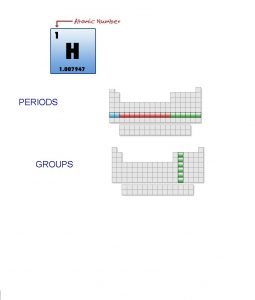
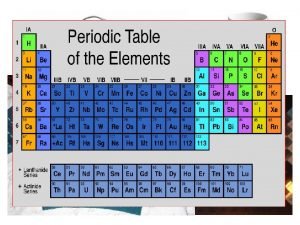
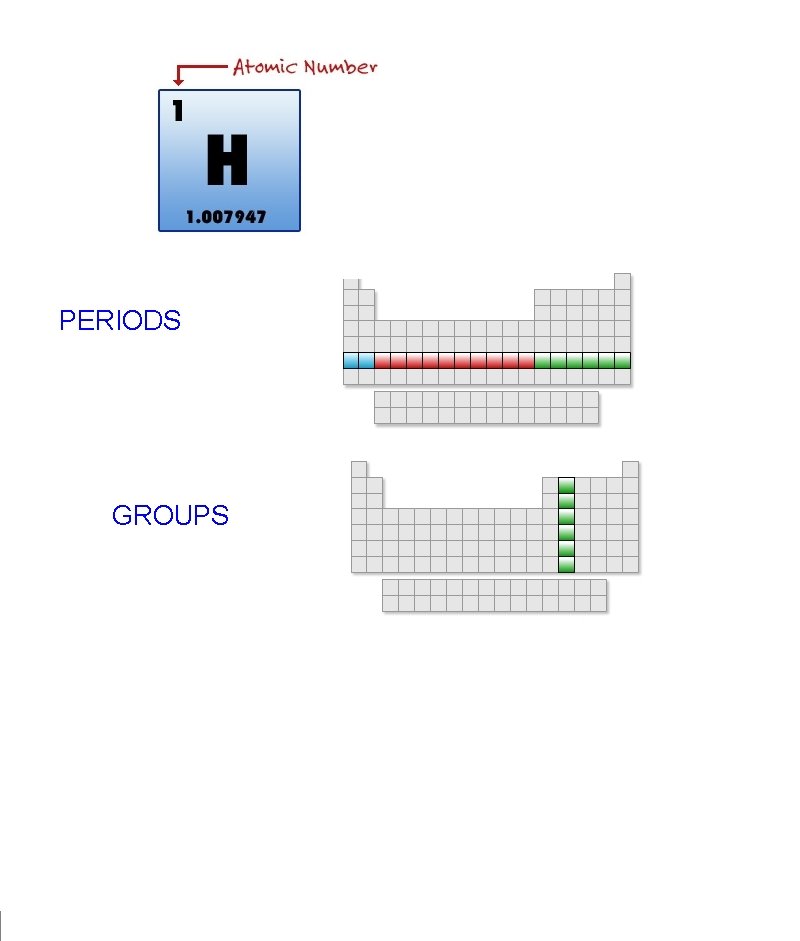
PERIODS GROUPS 1 23 periods 18 NAME THAT






- Slides: 18

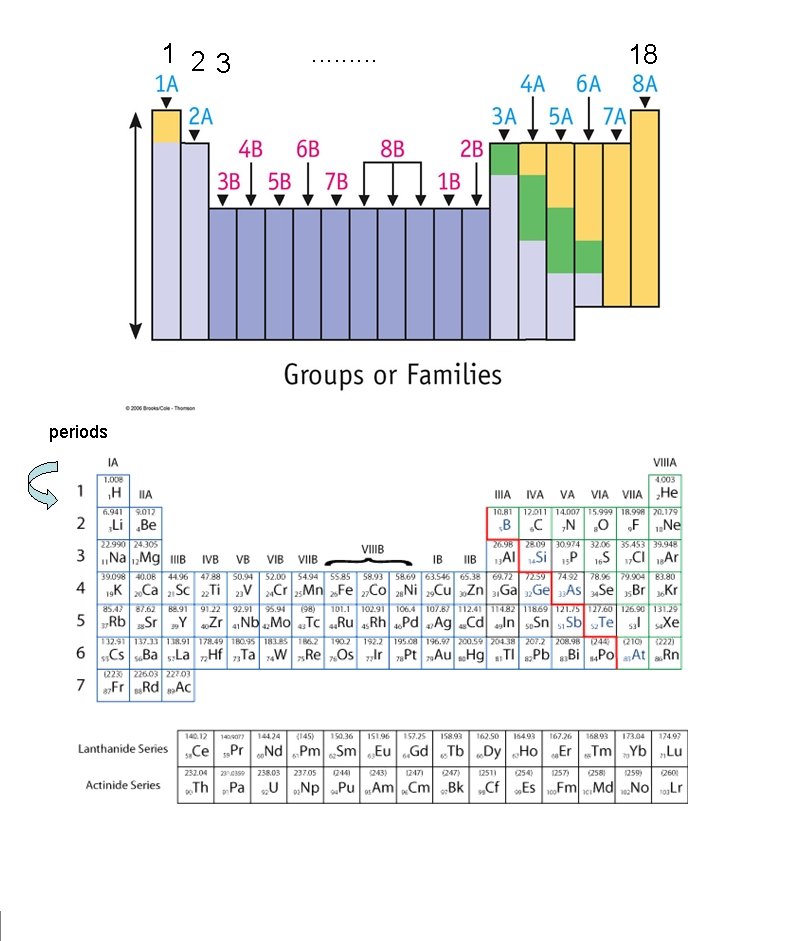
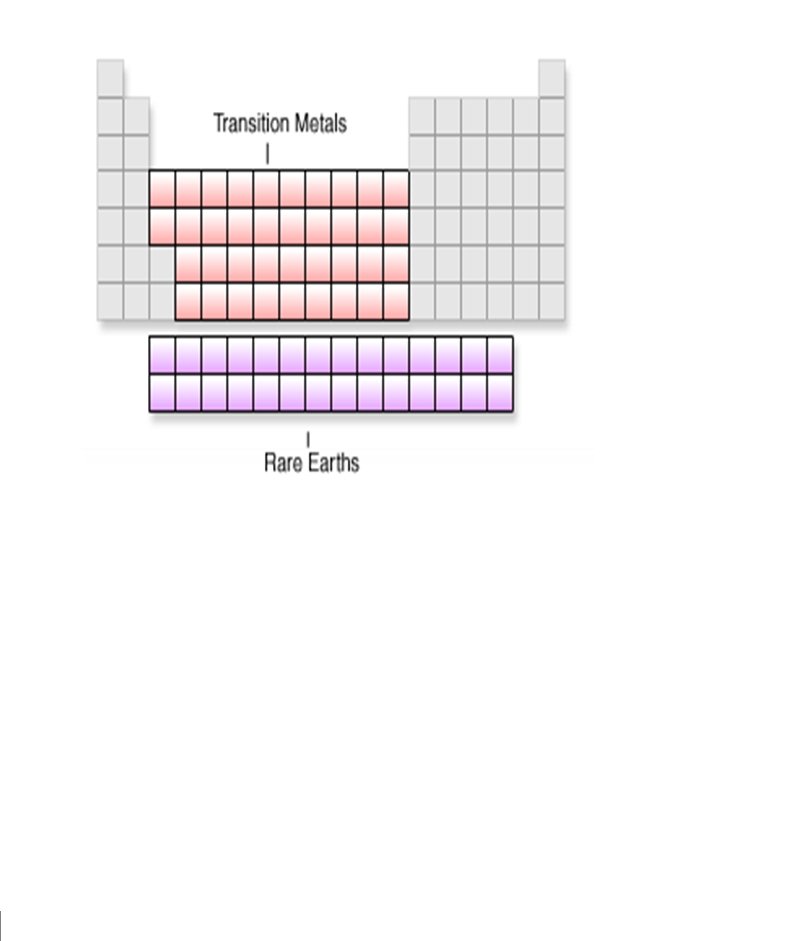
PERIODS GROUPS

1 23 periods . . 18

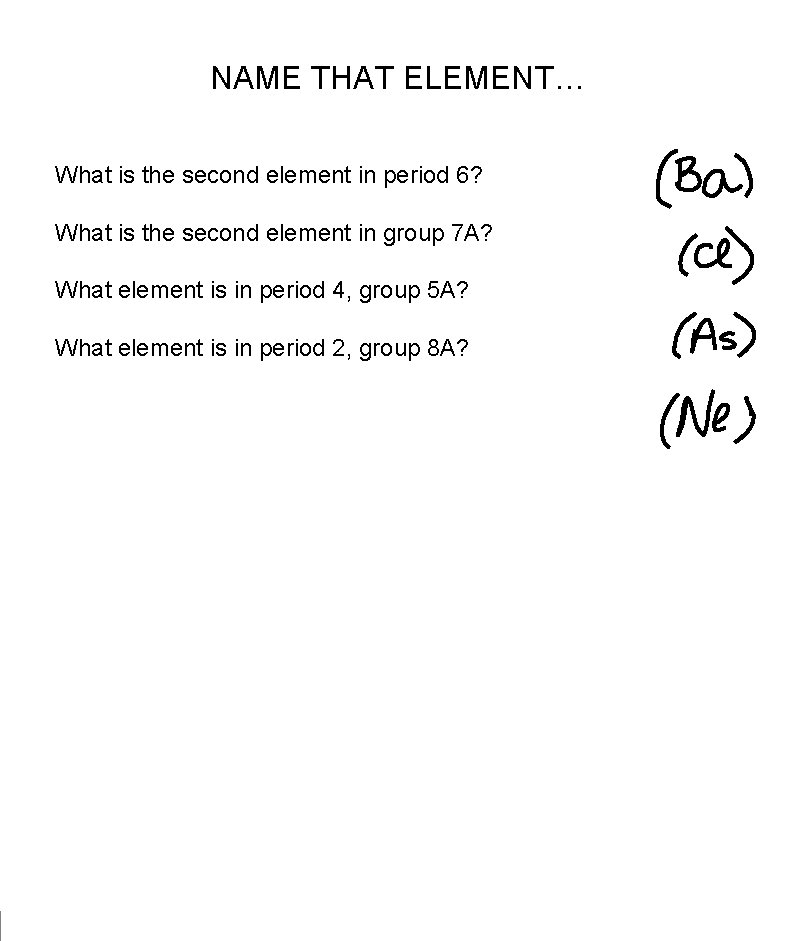
NAME THAT ELEMENT… What is the second element in period 6? What is the second element in group 7 A? What element is in period 4, group 5 A? What element is in period 2, group 8 A?

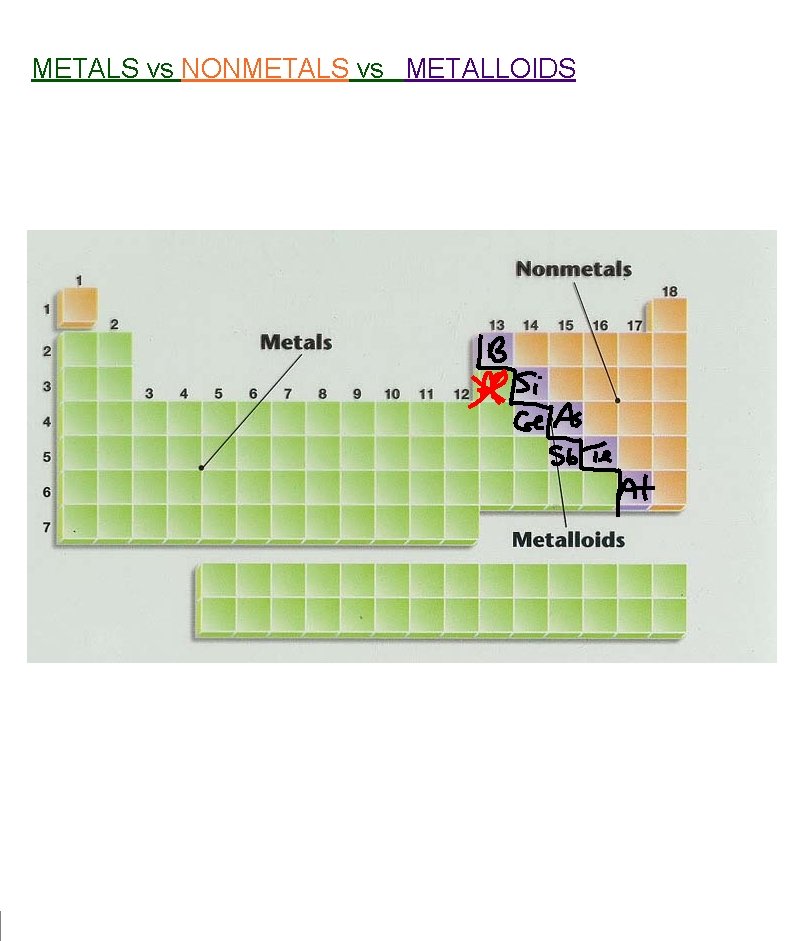
METALS vs NONMETALS vs METALLOIDS

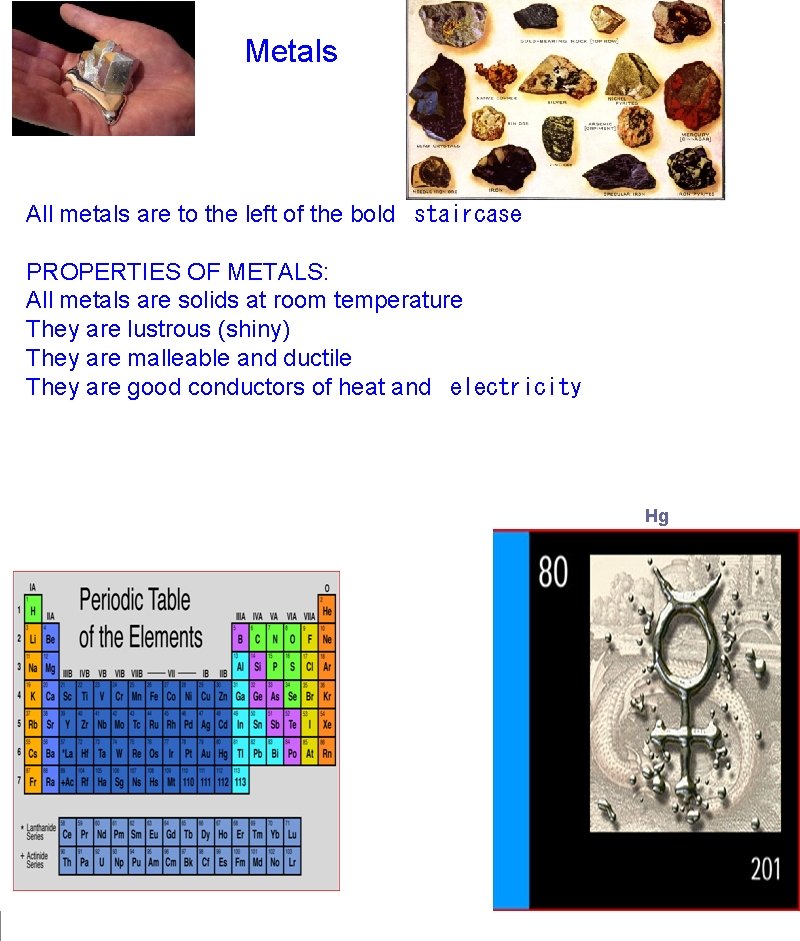
Metals All metals are to the left of the bold staircase PROPERTIES OF METALS: All metals are solids at room temperature They are lustrous (shiny) They are malleable and ductile They are good conductors of heat and electricity Hg (mercury)

Sulfur Nonmetals Chlorine gas Non-metals are to the right of the staircase. Nonmetals: generally nonlustrous, poor conductors of electricity Some gases (O, N, Cl); some are brittle solids (S); one is a fuming dark red liquid (Br)

Metalloids Found right along the staircase (except for Aluminum…it’s a metal) Have properties in-between metals and nonmetals Are “semi-conductors” (conduct a little bit) silicon


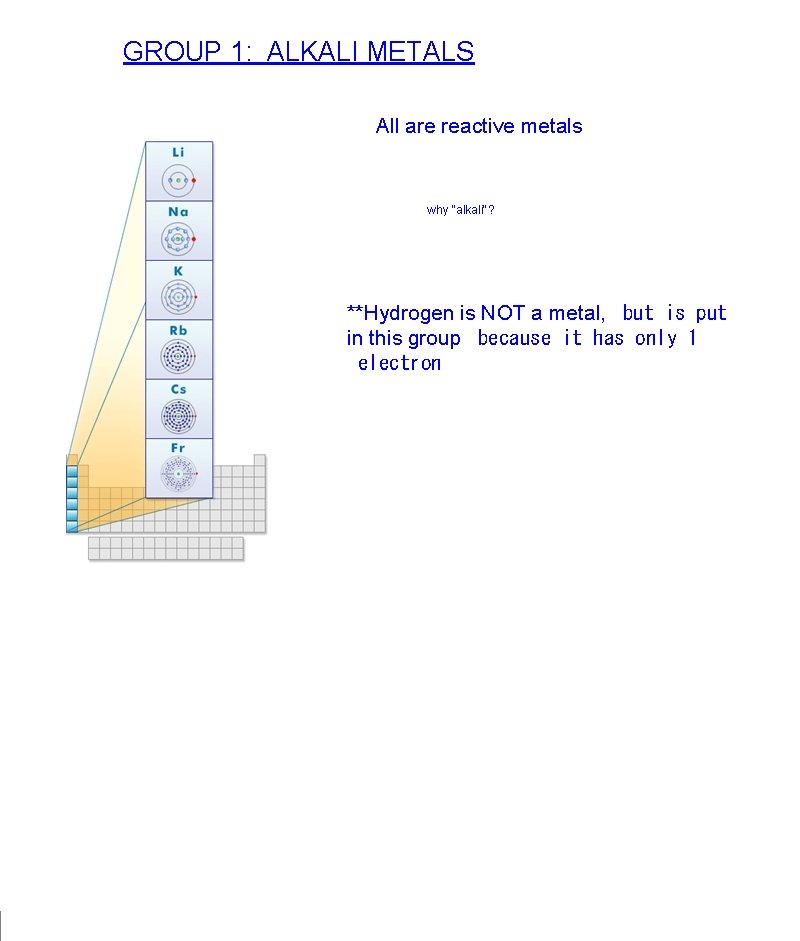
GROUP 1: ALKALI METALS All are reactive metals why "alkali"? **Hydrogen is NOT a metal, but is put in this group because it has only 1 electron

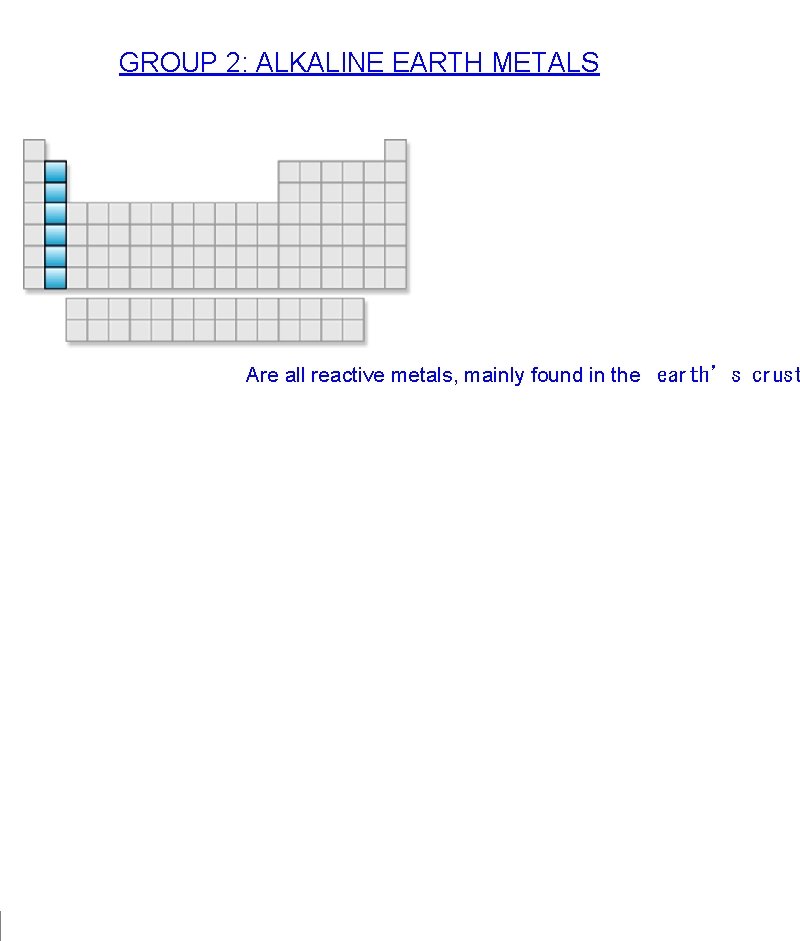
GROUP 2: ALKALINE EARTH METALS Are all reactive metals, mainly found in the earth’s crust

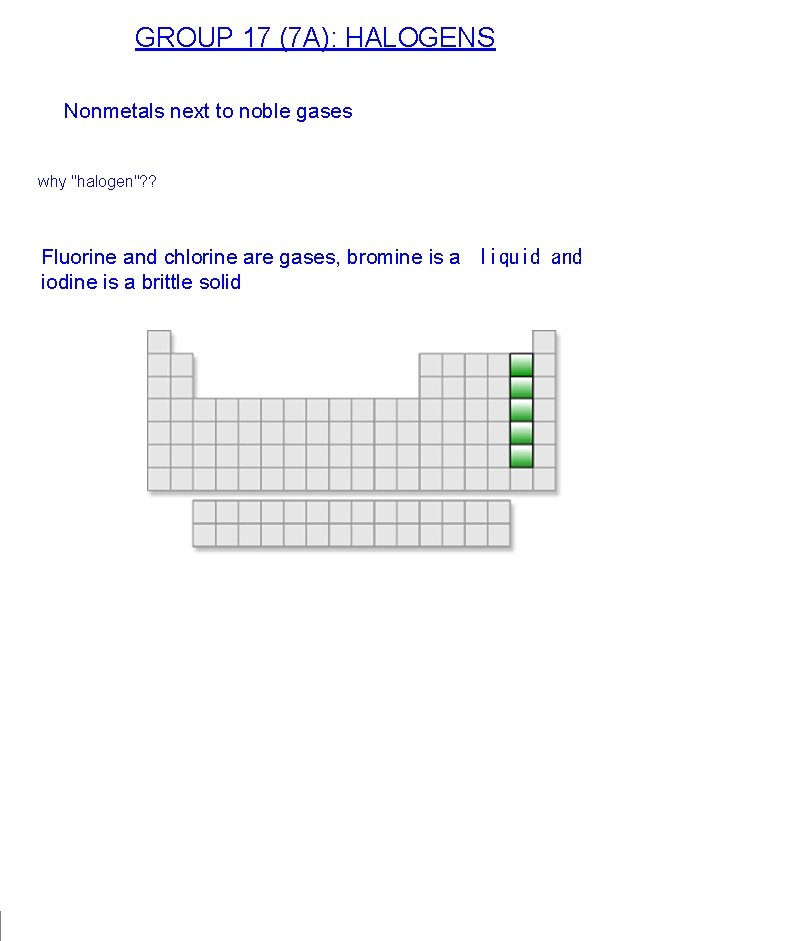
GROUP 17 (7 A): HALOGENS Nonmetals next to noble gases why "halogen"? ? Fluorine and chlorine are gases, bromine is a liquid and iodine is a brittle solid

GROUP 18 (8 A): NOBLE GASES!! Right side of the periodic table (on the end) All are gases at room temperature CHEMICALLY UNREACTIVE (Yet all other elements strive to be like them. . )

Which element? Period 6 halogen? Period 3 alkaline earth metal? Lightest (first) metal? Third heaviest alkali metal?

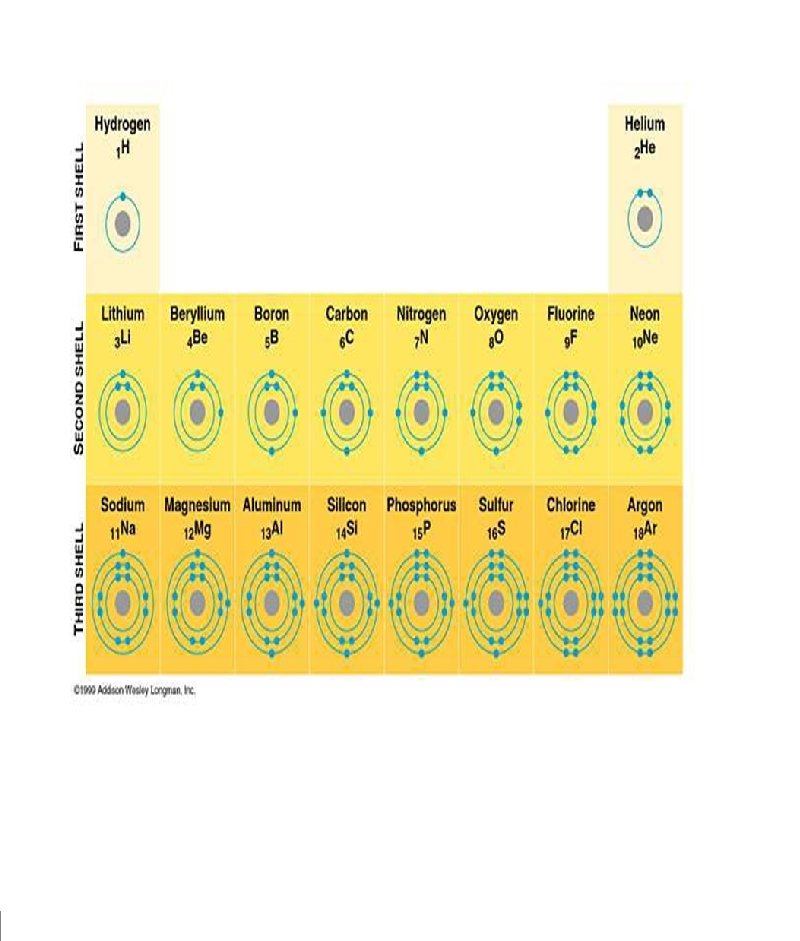
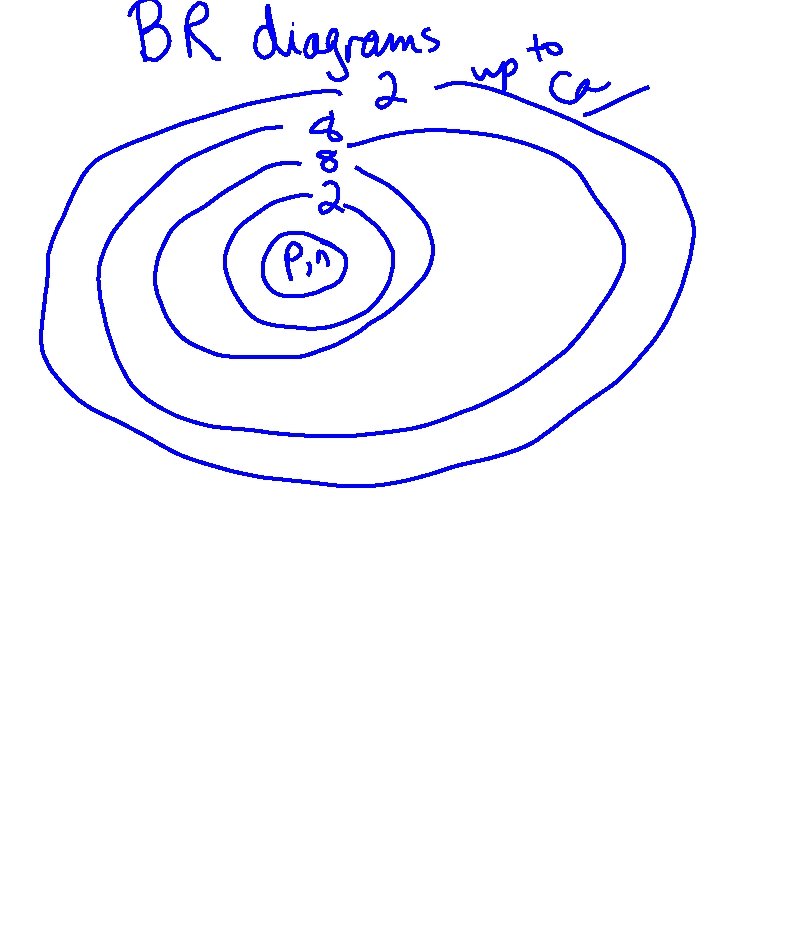
VALENCE ELECTRONS: THE ELECTRONS IN THE OUTERMOST RING (SHELL) *DETERMINES WHAT KIND OF ION WILL BE FORMED *THE ELECTRONS INVOLVED IN BONDING



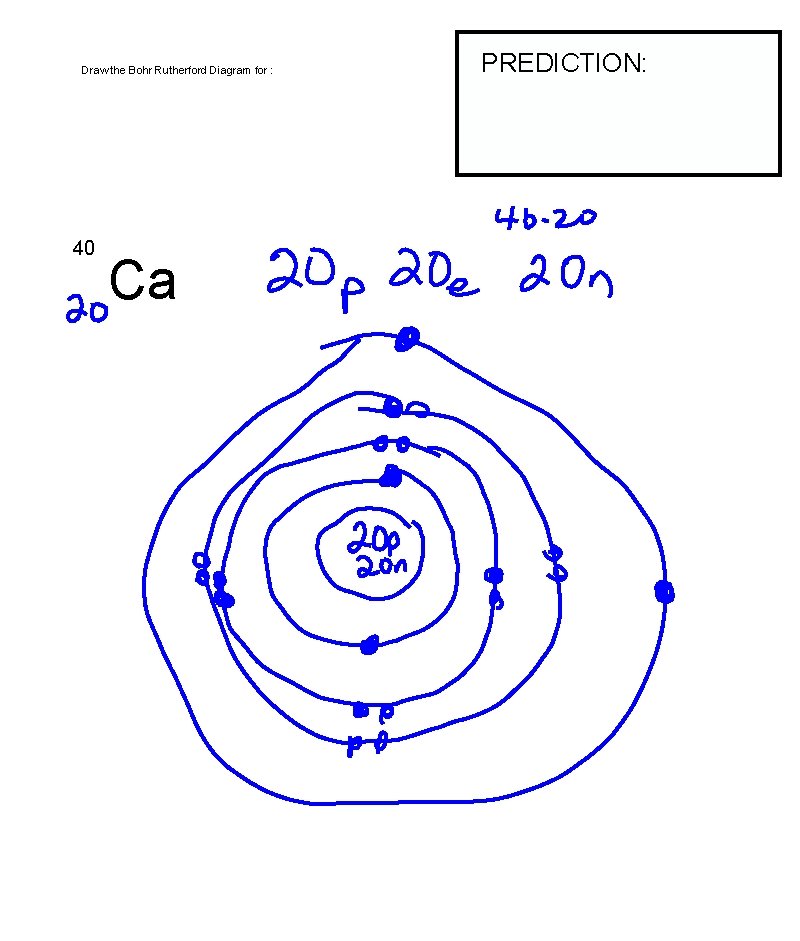
Draw the Bohr Rutherford Diagram for : 40 Ca PREDICTION:

Which element? Fills the 5 th ring? Puts two valence electrons in the 6 th ring? Half-fills the 2 nd ring? Puts 5 valence electrons in the 4 th ring ?
 Moseley periodic law
Moseley periodic law Groups and periods
Groups and periods Periodic table3
Periodic table3 Groups vs periods
Groups vs periods The periodic table displays the symbols and
The periodic table displays the symbols and Phân độ lown
Phân độ lown Block nhĩ thất độ 1
Block nhĩ thất độ 1 Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Chiến lược kinh doanh quốc tế của walmart
Chiến lược kinh doanh quốc tế của walmart Tìm vết của đường thẳng
Tìm vết của đường thẳng Con hãy đưa tay khi thấy người vấp ngã
Con hãy đưa tay khi thấy người vấp ngã Tôn thất thuyết là ai
Tôn thất thuyết là ai Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt How are ethnic groups and religious groups related
How are ethnic groups and religious groups related Name three lines
Name three lines Authors last name first name initial
Authors last name first name initial Name above every other name
Name above every other name