Periodicity Classification of the Elements u OBJECTIVES Explain

Periodicity
Classification of the Elements u. OBJECTIVES: • Explain why you can infer the properties of an element based on those of other elements in the periodic table.
Classification of the Elements u. OBJECTIVES: • Use electron configurations to classify elements as noble gases, main group elements, transition metals, or inner transition metals.

Periodic Table Revisited u Russian scientist Dmitri Mendeleev taught chemistry in terms of properties. u Mid 1800’s - molar masses of elements were known. u Wrote down the elements in order of increasing mass. u Found a pattern of repeating properties.

Mendeleev’s Table u Grouped elements in columns by similar properties in order of increasing atomic mass. u Found some inconsistencies - felt that the properties were more important than the mass, so switched order. u Also found some gaps. u Must be undiscovered elements. u Predicted their properties before they were found.

The modern table u Elements are still grouped by properties. u Similar properties are in the same column. u Order is by increasing atomic number. u Added a column of elements Mendeleev didn’t know about. u The noble gases weren’t found because they didn’t react with anything.
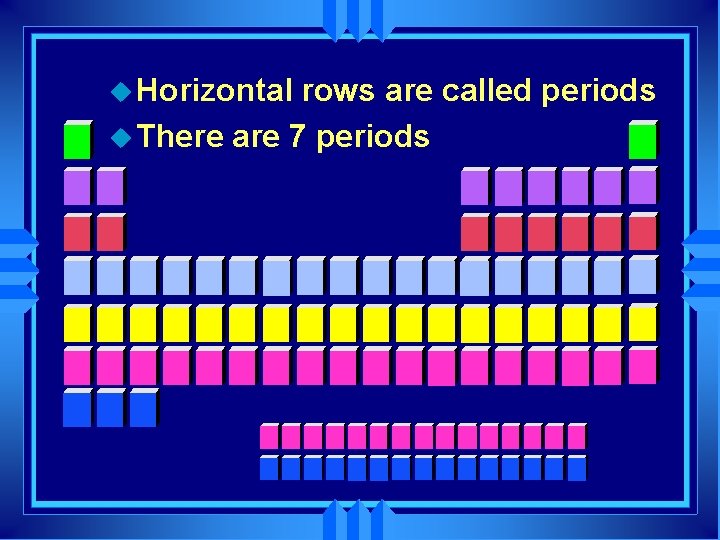
u Horizontal rows are called periods u There are 7 periods
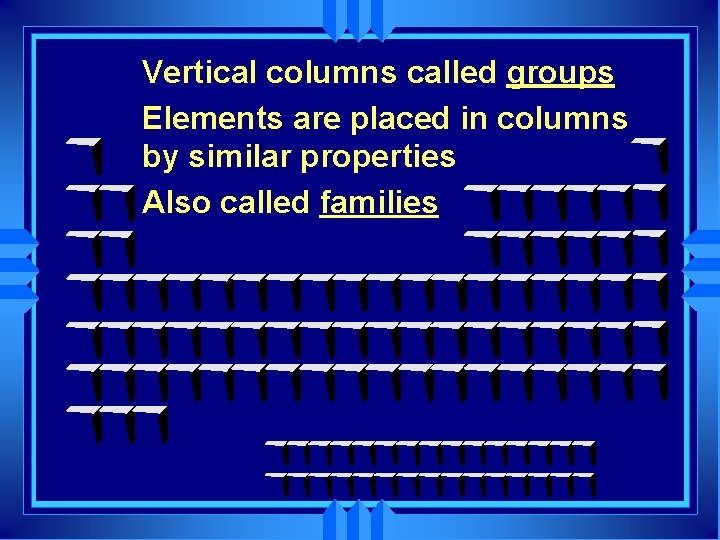
Vertical columns called groups Elements are placed in columns by similar properties Also called families
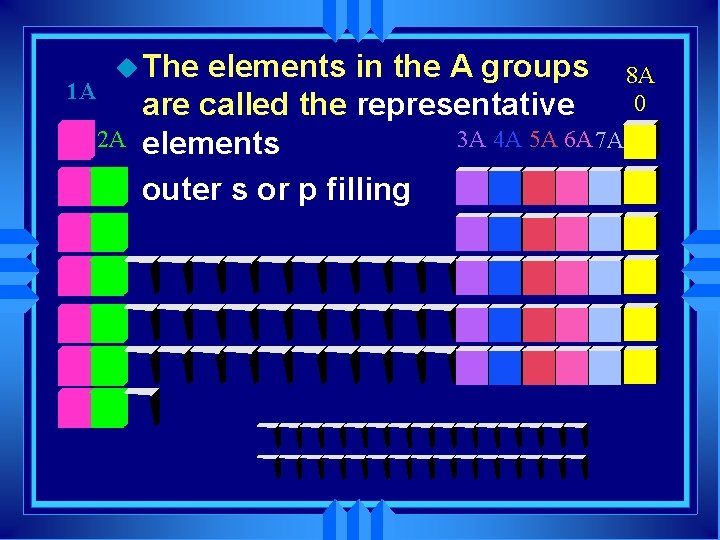
1 A u The 2 A elements in the A groups 8 A 0 are called the representative 3 A 4 A 5 A 6 A 7 A elements outer s or p filling
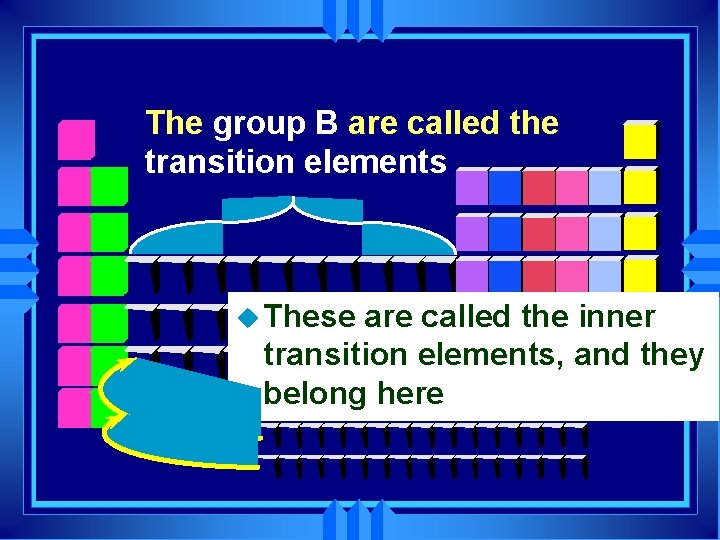
The group B are called the transition elements u These are called the inner transition elements, and they belong here
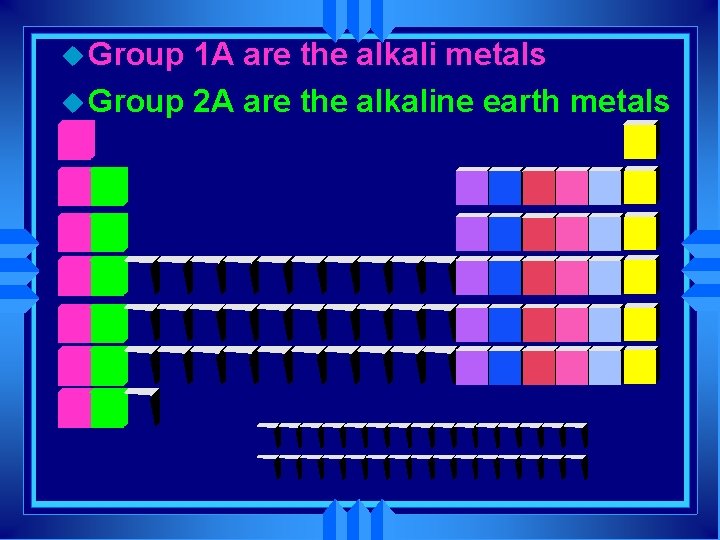
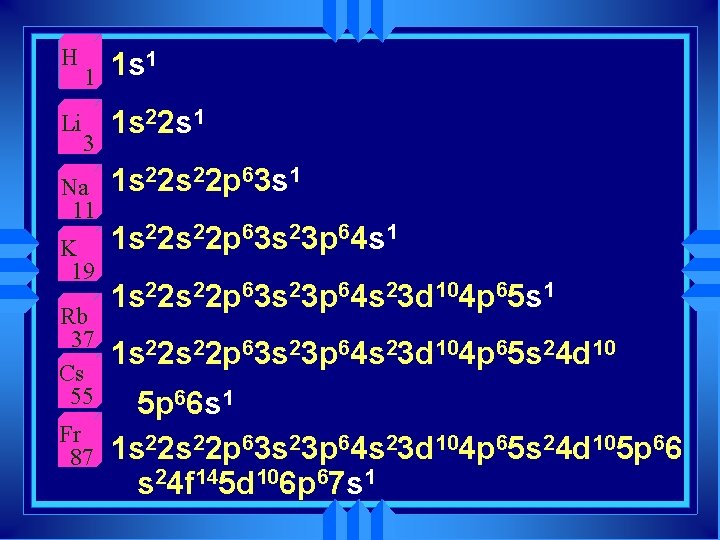
u Group 1 A are the alkali metals u Group 2 A are the alkaline earth metals
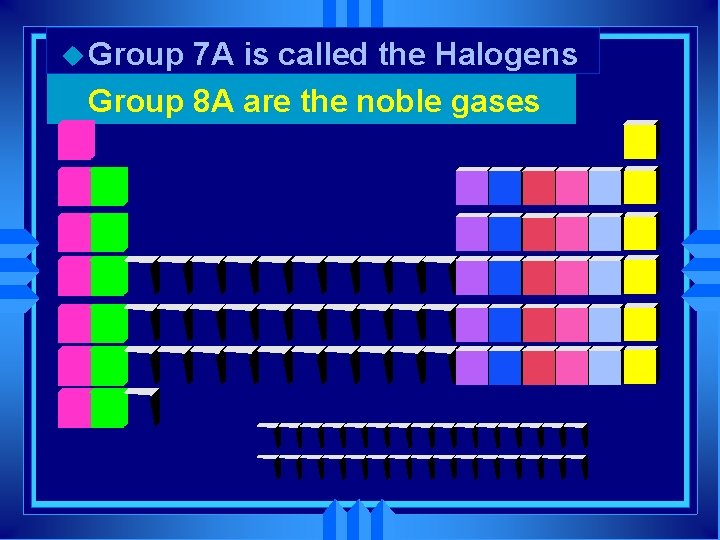
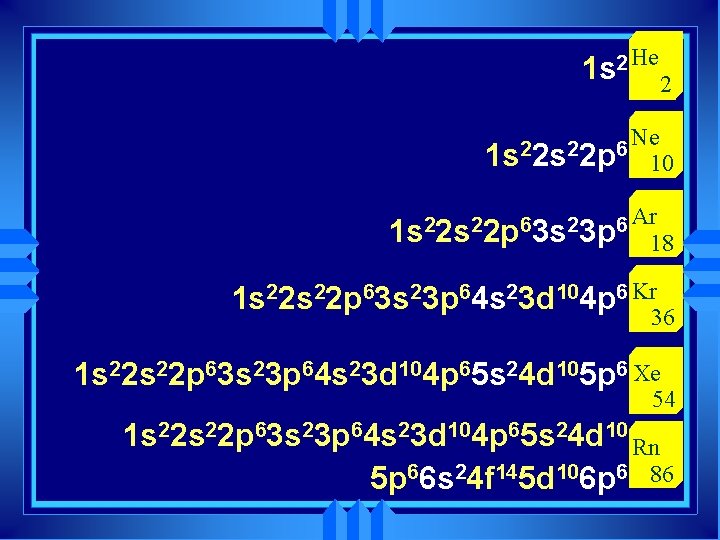
u Group 7 A is called the Halogens u Group 8 A are the noble gases

Why? u The part of the atom another atom sees is the electron cloud. u More importantly the outside orbitals. u The orbitals fill up in a regular pattern. u The outside orbital electron configuration repeats. u The properties of atoms repeat.
H Li 1 3 Na 11 K 19 Rb 37 Cs 55 Fr 87 1 s 1 1 s 22 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 1 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 10 5 p 66 s 1 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 105 p 66 s 24 f 145 d 106 p 67 s 1
1 s 2 He 2 1 s 22 p 6 Ne 10 Ar 2 2 6 1 s 2 s 2 p 3 s 3 p 18 1 s 22 p 63 s 23 p 64 s 23 d 104 p 6 Kr 36 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 105 p 6 Xe 54 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 10 Rn 5 p 66 s 24 f 145 d 106 p 6 86
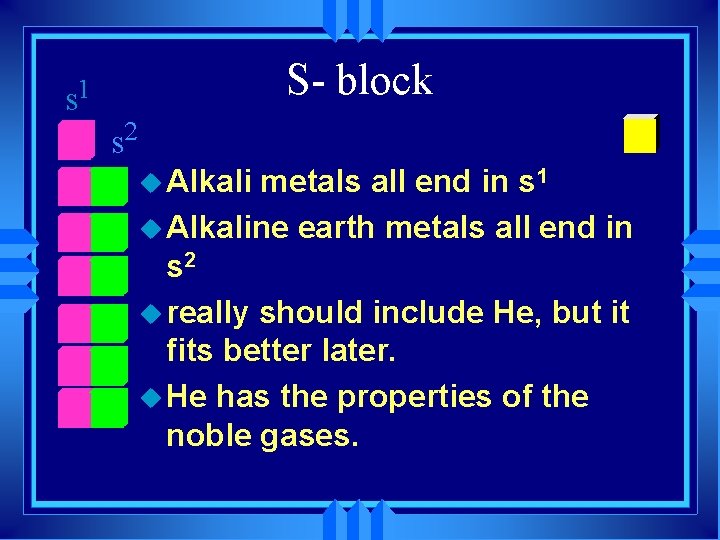
s 1 S- block s 2 u Alkali metals all end in s 1 u Alkaline earth metals all end in s 2 u really should include He, but it fits better later. u He has the properties of the noble gases.
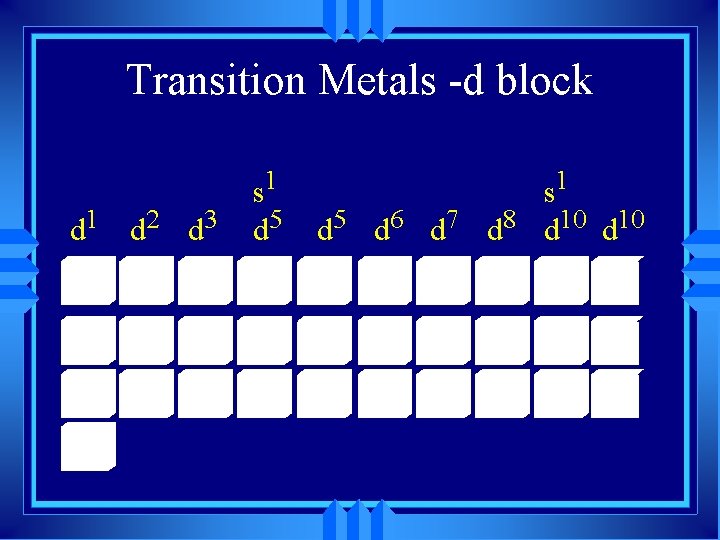
Transition Metals -d block 1 d 2 d 3 d s 1 5 6 7 8 10 10 d d d
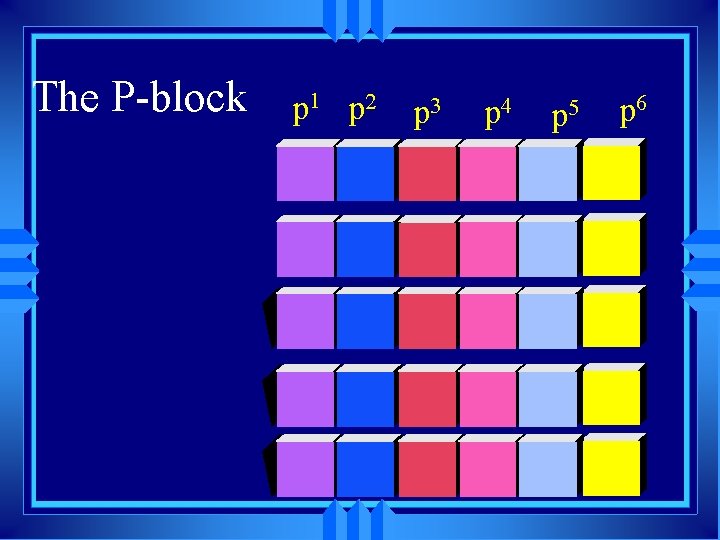
The P-block p 1 p 2 p 3 p 4 p 5 p 6
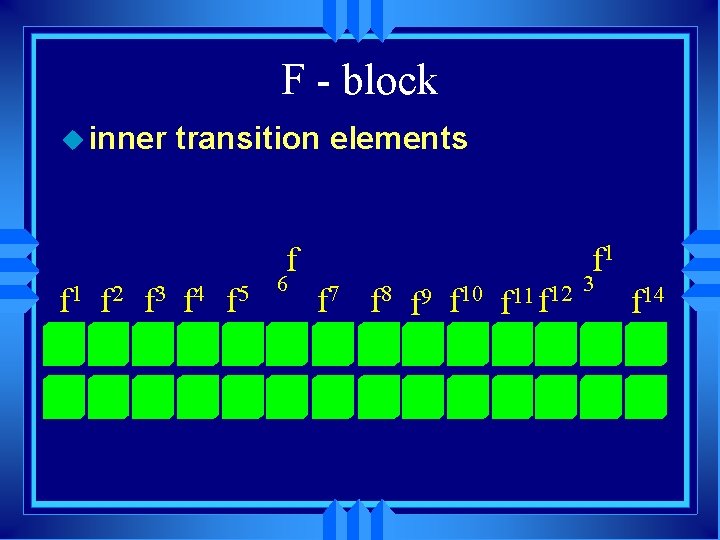
F - block u inner transition elements f 1 f 2 f 3 f 4 f 5 6 f 7 f 8 f 9 f 10 f 11 f 12 3 f 14
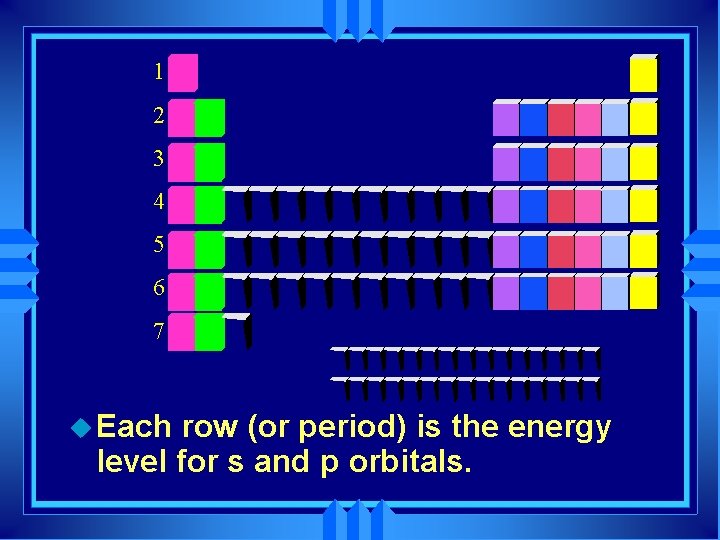
1 2 3 4 5 6 7 u Each row (or period) is the energy level for s and p orbitals.
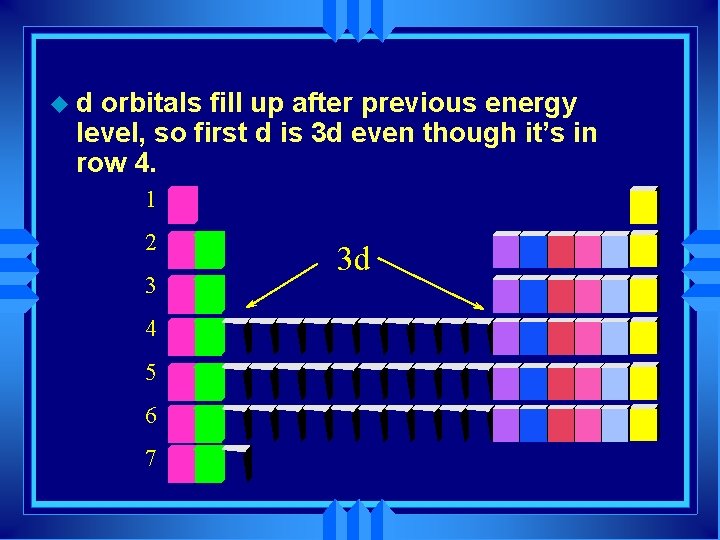
ud orbitals fill up after previous energy level, so first d is 3 d even though it’s in row 4. 1 2 3 4 5 6 7 3 d
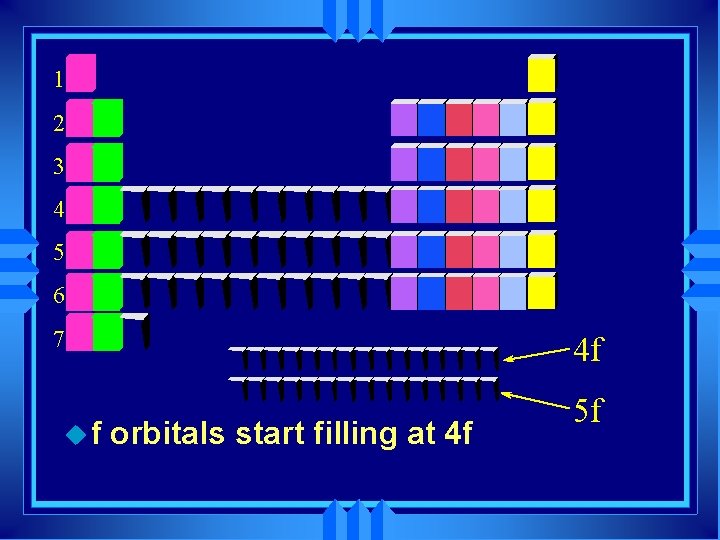
1 2 3 4 5 6 7 uf 4 f orbitals start filling at 4 f 5 f

Writing electron configurations the easy way

Electron Configurations repeat u. The shape of the periodic table is a representation of this repetition. u. When we get to the end of the column the outermost energy level is full. u. This is the basis for our shorthand.

The Shorthand u. Write symbol of the noble gas before the element, in [ ]. u. Then, the rest of the electrons. u. Aluminum’s full configuration: 1 s 22 p 63 s 23 p 1 uprevious noble gas Ne is: 1 s 22 p 6 uso, Al is: [Ne] 3 s 23 p 1

More examples u. Ge = 1 s 22 p 63 s 23 p 64 s 23 d 104 p 2 • Thus, Ge = [Ar] 4 s 23 d 104 p 2 u. Hf = 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 2 4 d 105 p 66 s 24 f 145 d 2 • Thus, Hf = [Xe]6 s 24 f 145 d 2
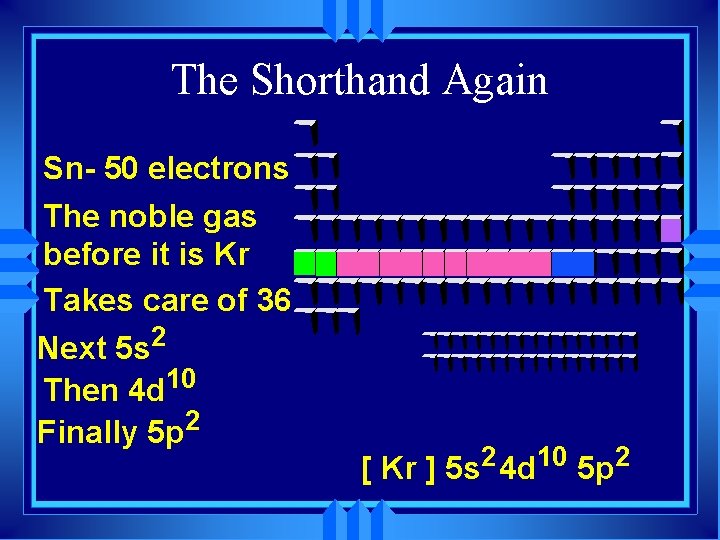
The Shorthand Again Sn- 50 electrons The noble gas before it is Kr Takes care of 36 Next 5 s 2 Then 4 d 10 Finally 5 p 2 [ Kr ] 5 s 2 4 d 10 5 p 2
- Slides: 27