PERIODICITY ATOMIC RADIUS The radius between the nuclei

PERIODICITY
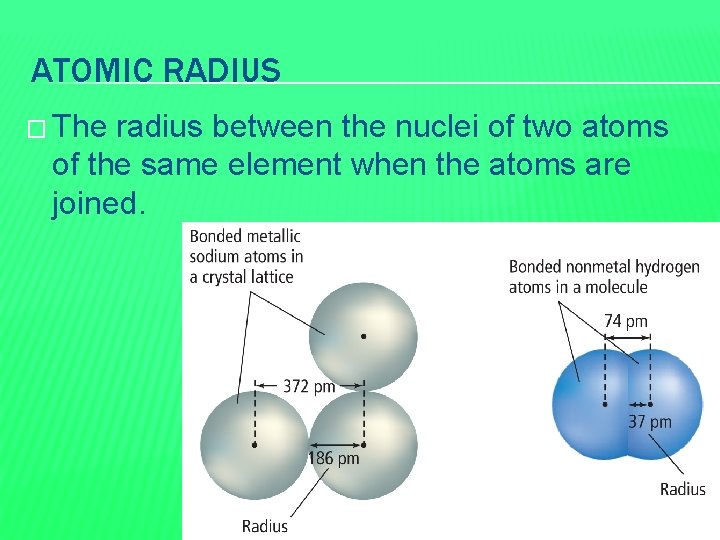
ATOMIC RADIUS � The radius between the nuclei of two atoms of the same element when the atoms are joined.
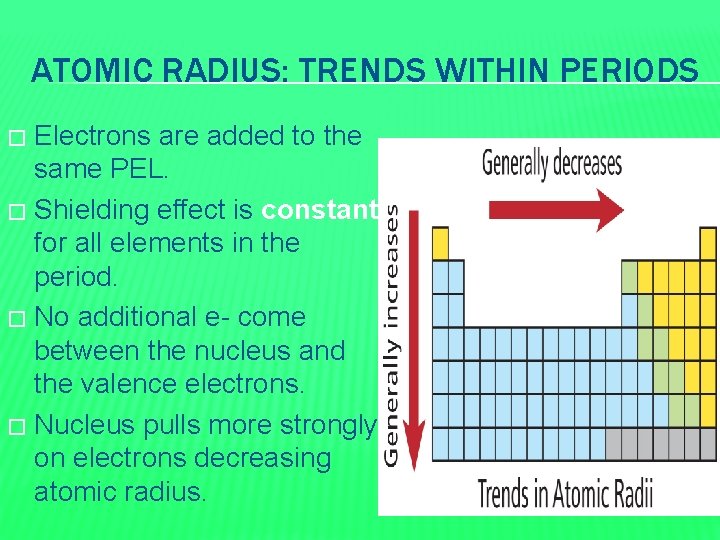
ATOMIC RADIUS: TRENDS WITHIN PERIODS Electrons are added to the same PEL. � Shielding effect is constant for all elements in the period. � No additional e- come between the nucleus and the valence electrons. � Nucleus pulls more strongly on electrons decreasing atomic radius. �
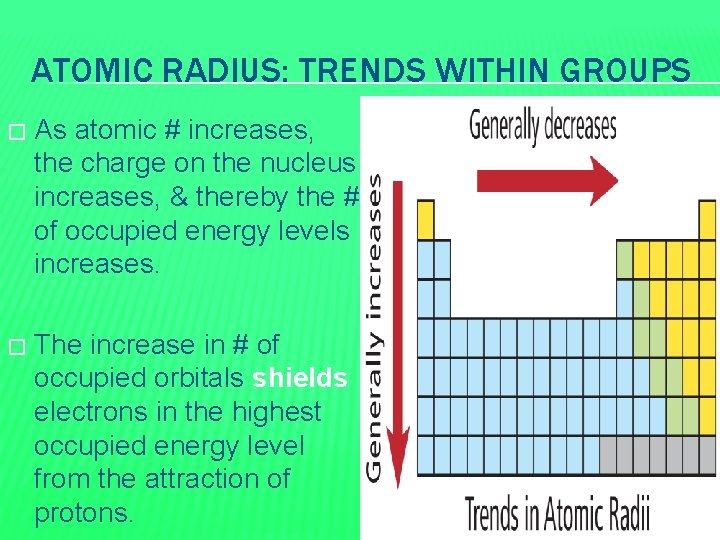
ATOMIC RADIUS: TRENDS WITHIN GROUPS � As atomic # increases, the charge on the nucleus increases, & thereby the # of occupied energy levels increases. � The increase in # of occupied orbitals shields electrons in the highest occupied energy level from the attraction of protons.
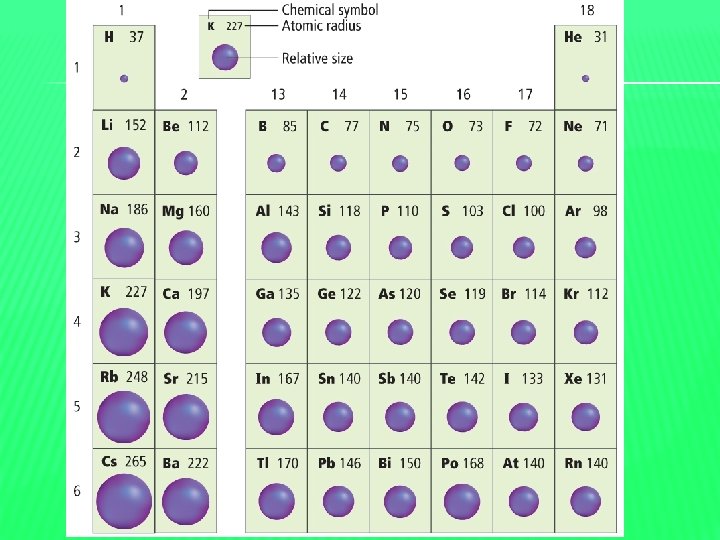

PRACTICE � Determine which element has the largest atomic radius and the smallest atomic radius: �Magnesium, Silicon, Sulfur, or Sodium �Largest – Sodium �Smallest – Sulfur
IONIZATION ENERGY � Ionization energy: energy required to remove an electron from a gaseous atom. � How strongly an atom’s nucleus holds on to its valence electrons.
IONIZATION ENERGY � High I. E. atom has strong hold on it’s electrons. � Low I. E. atom loses it’s outer electrons easily.
IONIZATION ENERGY: TRENDS WITHIN PERIODS � Increases from left to right across a period. � WHY? ? ? �Increased nuclear charge of each successive element produces an increased hold on the valence electrons.
IONIZATION ENERGY: TRENDS WITHIN GROUPS � Decreases as you move down a group. � WHY? ? ? �Atomic size increases as you move down a group. �Less energy is required to remove valence electrons farther from the nucleus because there is more shielding.

PRACTICE � For each of the following pairs, predict which atom has the higher first ionization energy. � Mg, Na �Mg � S, O �O � Ca, Ba �Ca
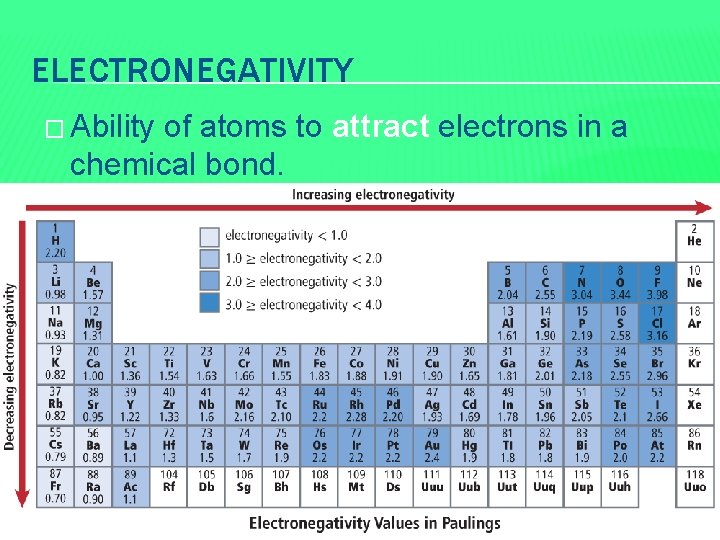
ELECTRONEGATIVITY � Ability of atoms to attract electrons in a chemical bond.
ACROSS A PERIOD � Electronegativity increases. � Small atoms tend to gain electrons because they want a full outer valence shell. They want 8.
DOWN A GROUP � Electronegativity decreases going down a group because of the increase in size of the atoms. Large atoms tend to lose electrons because there is increased shielding.

PRACTICE � For each of the following pairs, predict which atom has the higher electronegativity. � Mg, Na �Mg � Na, Al � Cl, I �Cl
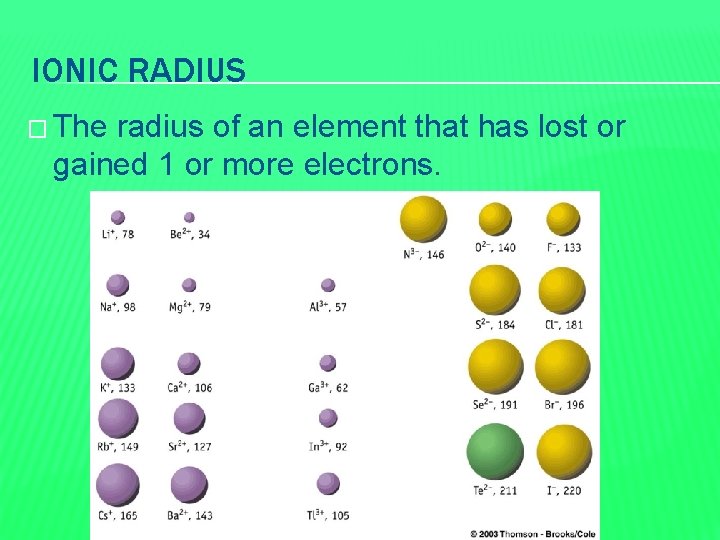
IONIC RADIUS � The radius of an element that has lost or gained 1 or more electrons.
FACTORS THAT INFLUENCE IONIC SIZE � Positive ions – formed when atoms lose electrons. The resulting cation is always smaller than its parent atom. � Negative ions – formed when atoms gain electrons. The resulting anion is always larger than its parent atom.
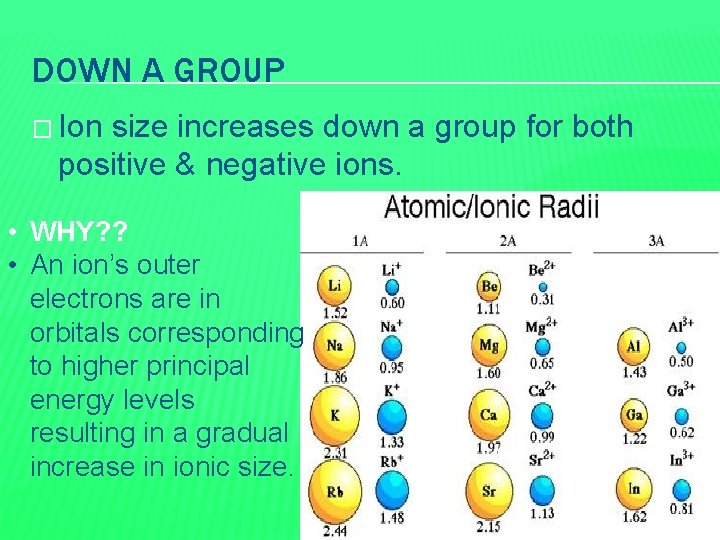
DOWN A GROUP � Ion size increases down a group for both positive & negative ions. • WHY? ? • An ion’s outer electrons are in orbitals corresponding to higher principal energy levels resulting in a gradual increase in ionic size.
ACROSS A PERIOD � Contains elements that form both cations and anions so there are variances. � For positive ions– decreases to the right � For negative ions – decreases to the right
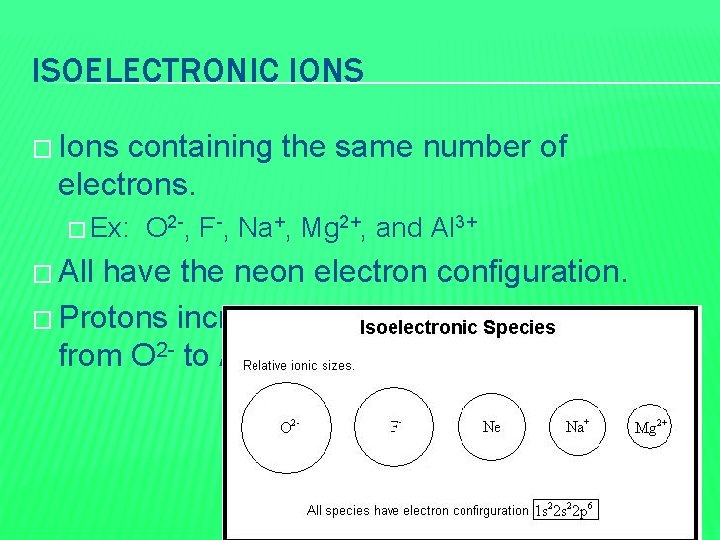
ISOELECTRONIC IONS � Ions containing the same number of electrons. � Ex: � All O 2 -, F-, Na+, Mg 2+, and Al 3+ have the neon electron configuration. � Protons increases from 8 to 13 as you go from O 2 - to Al 3+
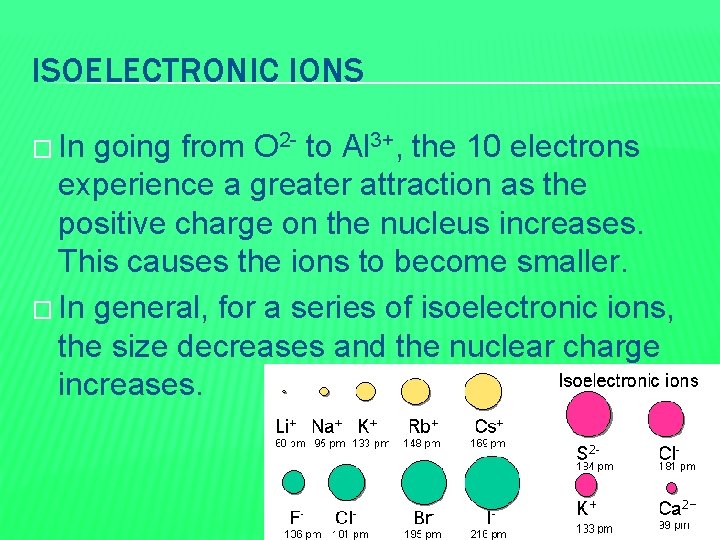
ISOELECTRONIC IONS � In going from O 2 - to Al 3+, the 10 electrons experience a greater attraction as the positive charge on the nucleus increases. This causes the ions to become smaller. � In general, for a series of isoelectronic ions, the size decreases and the nuclear charge increases.

EXAMPLE � Which of the ions listed below are isoelectronic with krypton? � Ag+, Br -, Cd 2+, Sc 3+, Se 2 -, Sr 2+, Ti 2+, Zn 2+ � Br -, Se 2 -, Sr 2+

EXAMPLES � Which species of each pair has the larger radius? 1. 2. 3. 4. Mg or Mg 2+ O or O 2 K+ or Cl. P 3 - or S 2 -
ELECTRON AFFINITY � The energy given off when a neutral atom in the gas phase gains an extra electron to form an anion. � In other words, the neutral atom's likelihood of gaining an electron.

ELECTRON AFFINITY � Best way to think about electron affinity: � A measure of the attraction between an incoming electron and the nucleus. � The stronger the attraction = the more energy released.

DOWN A GROUP � Electron affinity decreases � WHY? ? ? � The electrons are placed in higher energy levels further from the nucleus, thus there is a decrease in the pull of the nucleus due to shielding. � Therefore, there is not a strong attraction between the nucleus and the outer electrons & electron affinity decreases.

ACROSS A PERIOD � Electron affinity increases. � WHY? ? ? � Electrons are added to the same PEL. There is no shielding, therefore, there is a strong attraction between the nucleus and outer electrons.
EXAMPLE � Arrange the following elements in order of increasing electron affinity: P, Na, Ar, Cl � Ar < Na < P < Cl � Arrange the following elements in order of decreasing electron affinity: Cu, K, Ni, Br � Br > Cu > Ni > K
- Slides: 28