Periodic Trends Use these 3 periodic things to


- Slides: 14

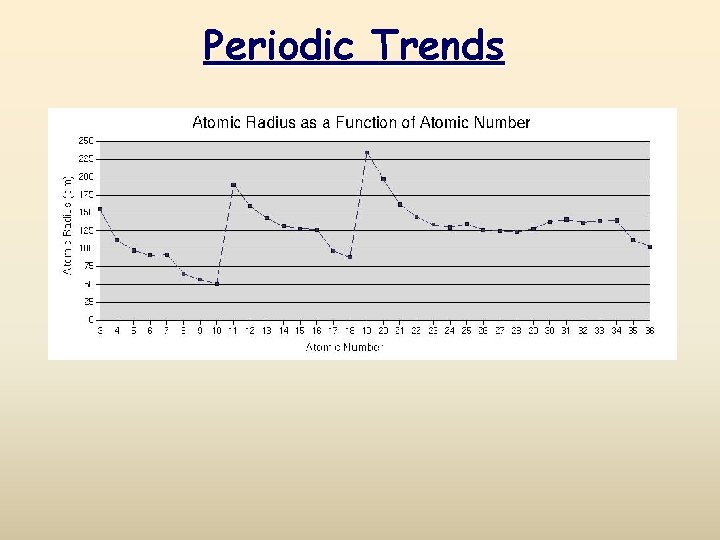
Periodic Trends

Use these 3 periodic things to explain table trends • Use Zeff to justify trends across a period (Zeff – effective nuclear charge or amount of p+ in nucleus) • Use increased distance to justify trends down a group • ALWAYS mention BOTH of the atoms or ions in the question when stating your answer

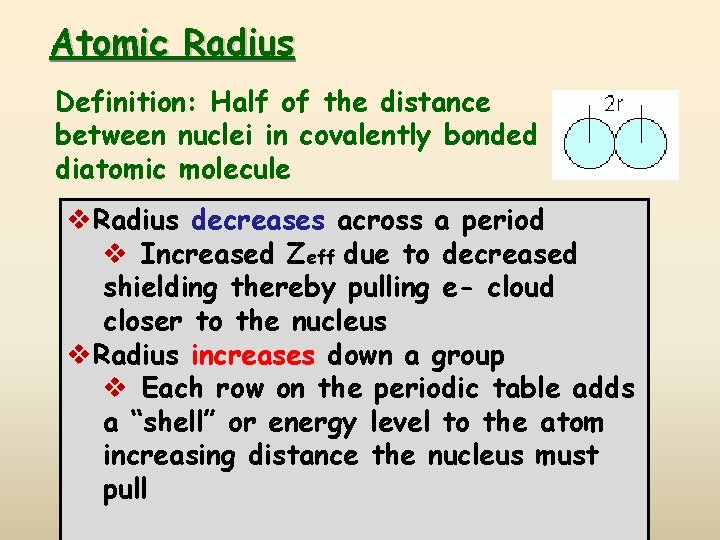
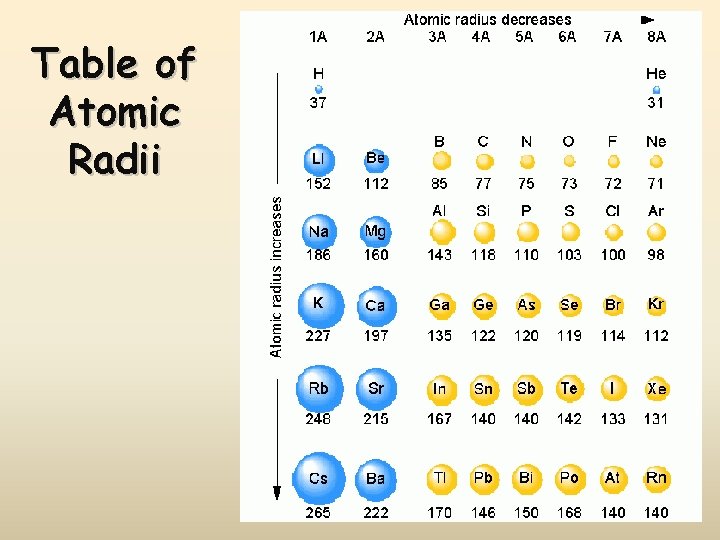
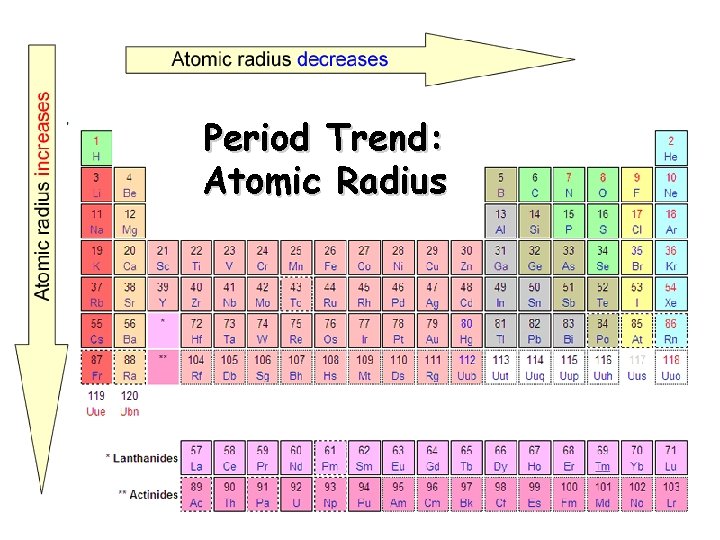
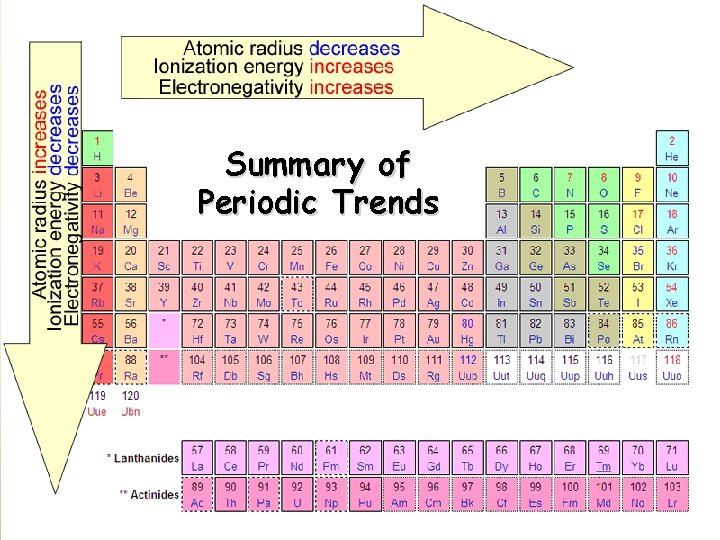
Atomic Radius Definition: Half of the distance between nuclei in covalently bonded diatomic molecule v. Radius decreases across a period v Increased Zeff due to decreased shielding thereby pulling e- cloud closer to the nucleus v. Radius increases down a group v Each row on the periodic table adds a “shell” or energy level to the atom increasing distance the nucleus must pull

Table of Atomic Radii

Period Trend: Atomic Radius

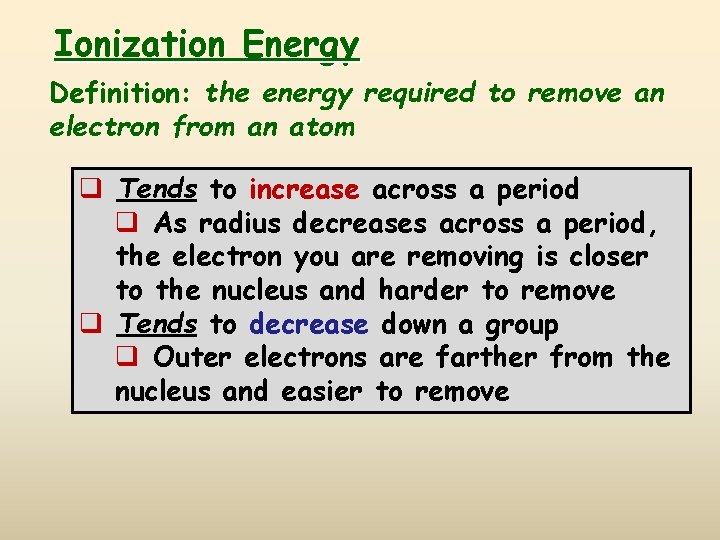
Ionization Energy Definition: the energy required to remove an electron from an atom q Tends to increase across a period q As radius decreases across a period, the electron you are removing is closer to the nucleus and harder to remove q Tends to decrease down a group q Outer electrons are farther from the nucleus and easier to remove

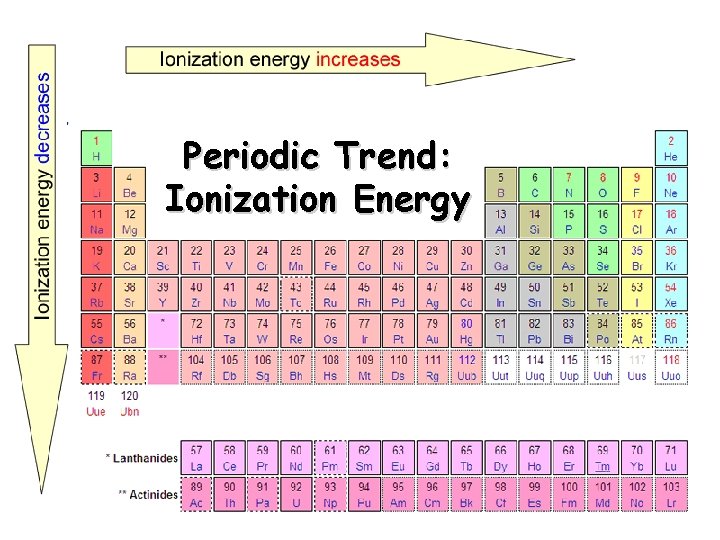
Periodic Trend: Ionization Energy

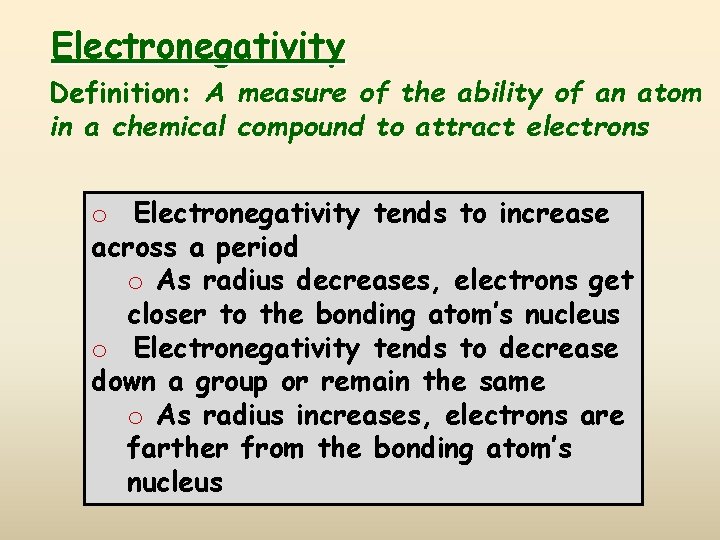
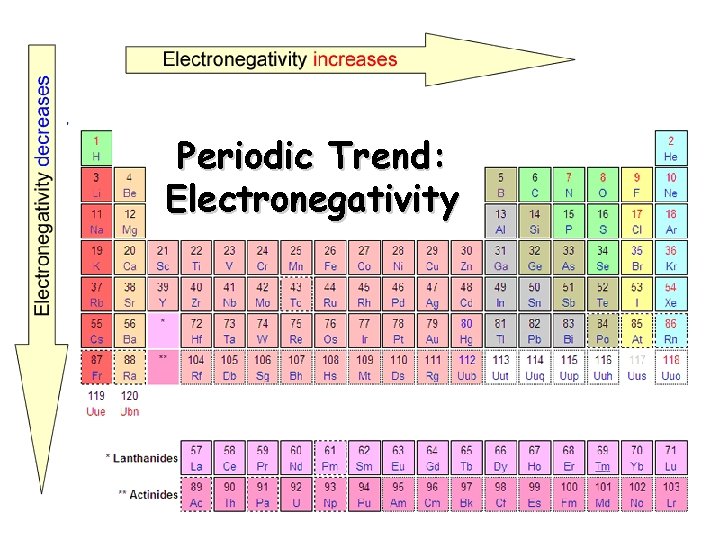
Electronegativity Definition: A measure of the ability of an atom in a chemical compound to attract electrons o Electronegativity tends to increase across a period o As radius decreases, electrons get closer to the bonding atom’s nucleus o Electronegativity tends to decrease down a group or remain the same o As radius increases, electrons are farther from the bonding atom’s nucleus

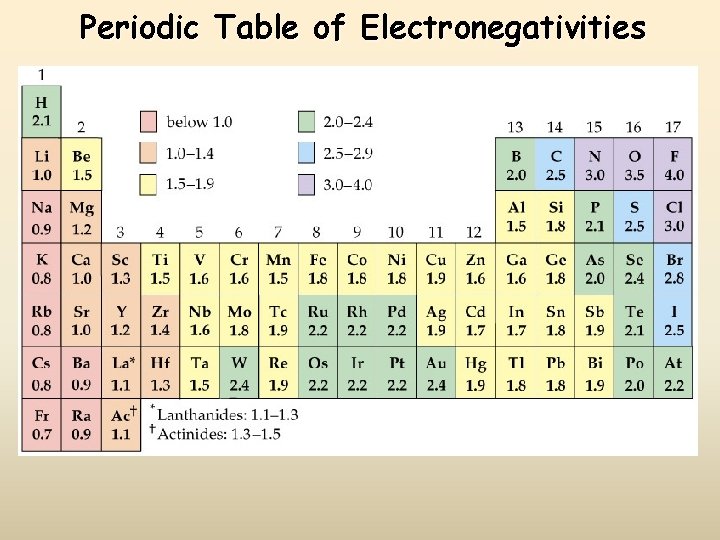
Periodic Table of Electronegativities

Periodic Trend: Electronegativity

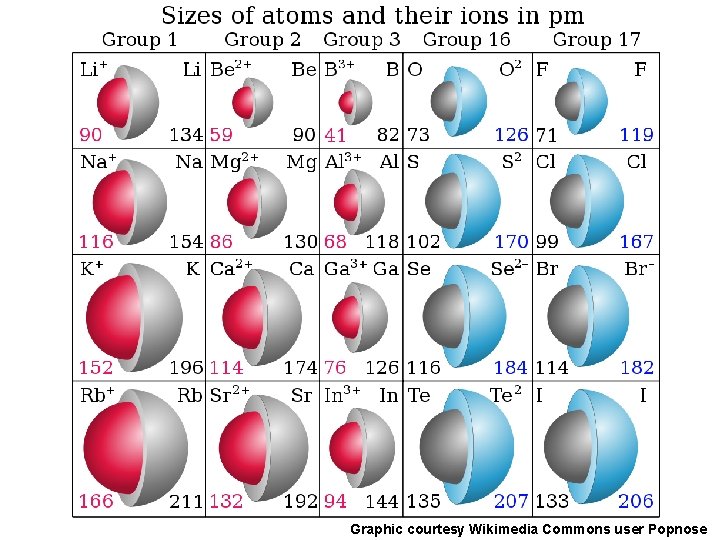
Ionic Radius Definition: distance from the nucleus to the outer edge of the electron cloud in a charged ion. (same general trend as atomic radius) Electron Affinity Definition: the energy gained or lost with the addition of an electron to a gaseous atom or ion.

Summary of Periodic Trends

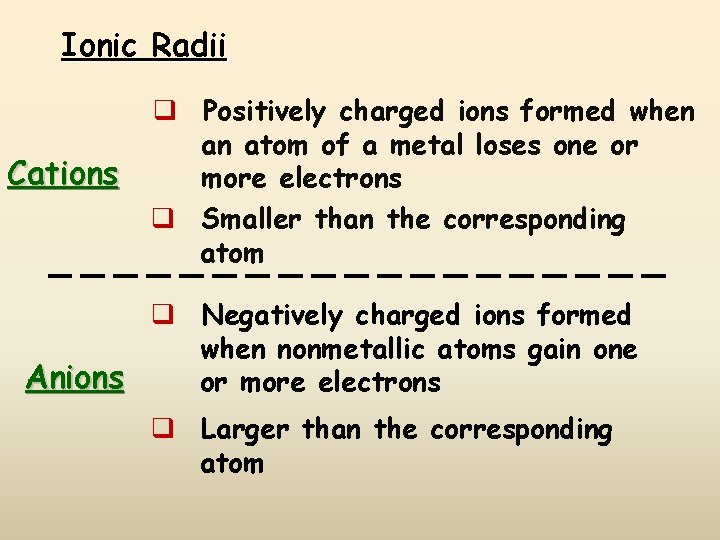
Ionic Radii Cations q Positively charged ions formed when an atom of a metal loses one or more electrons q Smaller than the corresponding atom q Negatively charged ions formed when nonmetallic atoms gain one Anions or more electrons q Larger than the corresponding atom

Graphic courtesy Wikimedia Commons user Popnose