Periodic Trends The size of the atoms increase



- Slides: 6

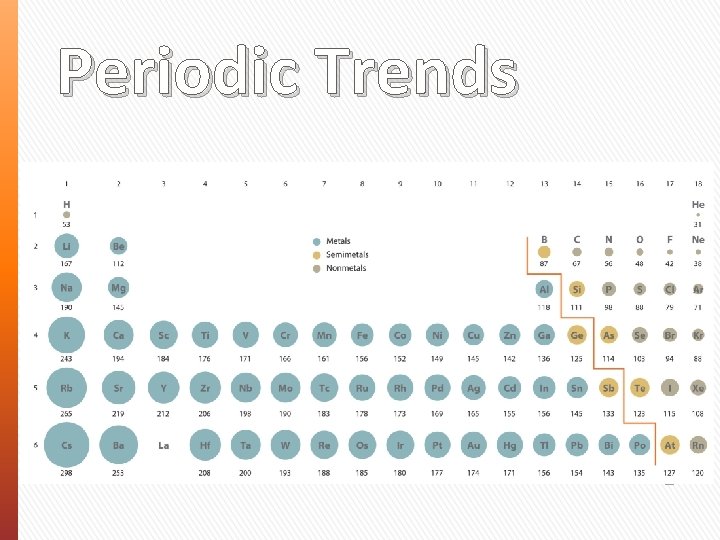
Periodic Trends » The size of the atoms increase down the group There’s an increase in the number of shells going down. » The size of the atoms decrease from left to right of a period. This is because the more protons and electrons, the more compact the atom becomes.

Periodic Trends

Periodic Trends » Group I (1): Alkali Metals – They are very reactive and naturally react with water to form basic alkaline solutions. » Group II (2): Alkaline Earth Metals – Abundant in the earth and also react to form basic alkaline solutions. » Group 3 -12: Transition metals – Most of the strong metals » Group III-VIII (13 -18): Non-metals » Group VII (17): Halogens – Reactive non-metals. » Group VIII (18): Noble gas – Stable & non-reactive

Ions The number of electrons an atom contains is not fixed. Atoms are most stable when they have 8 electrons in their outer shell (except for the first shell). Atoms can gain or lose electrons by interacting with other atoms to become stable. When an atom gains or loses electrons they become charged (+ or -). This is called an Ion Cation = Positive Ion Anion = Negative Ion

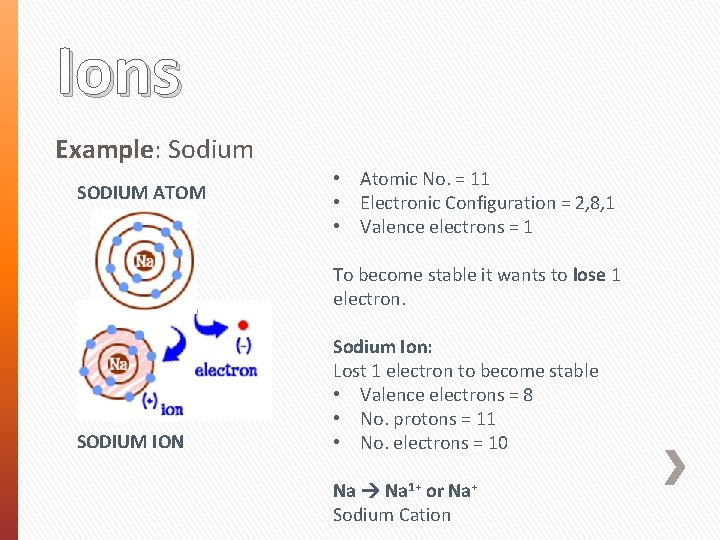
Ions Example: Sodium SODIUM ATOM • Atomic No. = 11 • Electronic Configuration = 2, 8, 1 • Valence electrons = 1 To become stable it wants to lose 1 electron. SODIUM ION Sodium Ion: Lost 1 electron to become stable • Valence electrons = 8 • No. protons = 11 • No. electrons = 10 Na 1+ or Na+ Sodium Cation

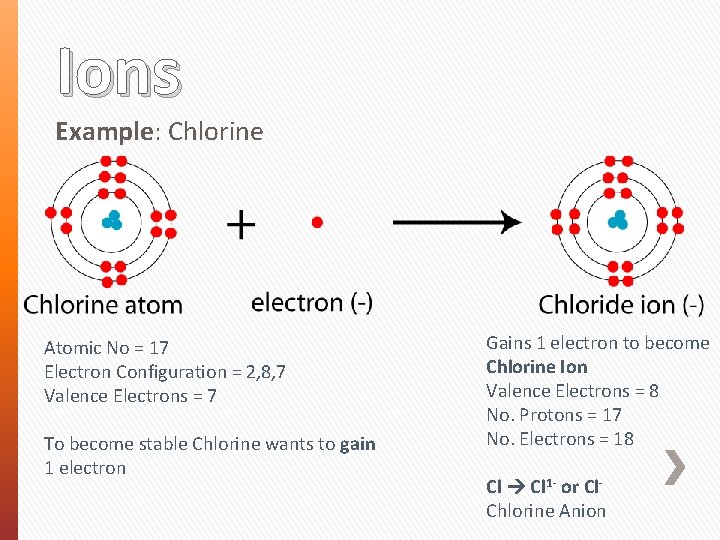
Ions Example: Chlorine Atomic No = 17 Electron Configuration = 2, 8, 7 Valence Electrons = 7 To become stable Chlorine wants to gain 1 electron Gains 1 electron to become Chlorine Ion Valence Electrons = 8 No. Protons = 17 No. Electrons = 18 Cl 1 - or Cl. Chlorine Anion