Periodic Trends Reactivity Reactivity of Metals increases as









- Slides: 17

Periodic Trends

Reactivity Ø Reactivity of Metals increases as you move down a group. l (Group 1 most reactive) Ø Reactivity of Non-Metals increases as you move up a group. l (Group 17 most reactive)

Metallic Nature Ø Elements that are closest to the bottom left are the most metallic. l Metals are: conductive, lustrous, malleable, ductile, solid at STP, reactive with acids etc. Ø Elements that are closest to the upper right are the least metallic. l Non-Metals are more varied in their properties, but they tend to be: non-conductive, brittle, and non reactive with acids. Many are gases at room temperature.

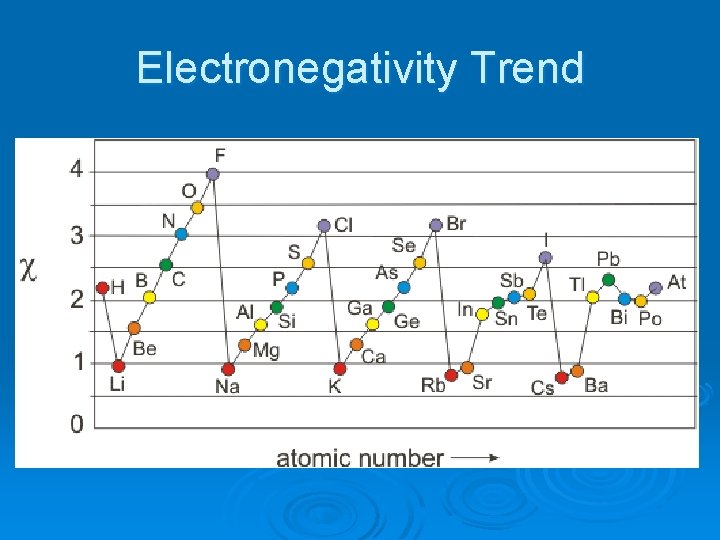
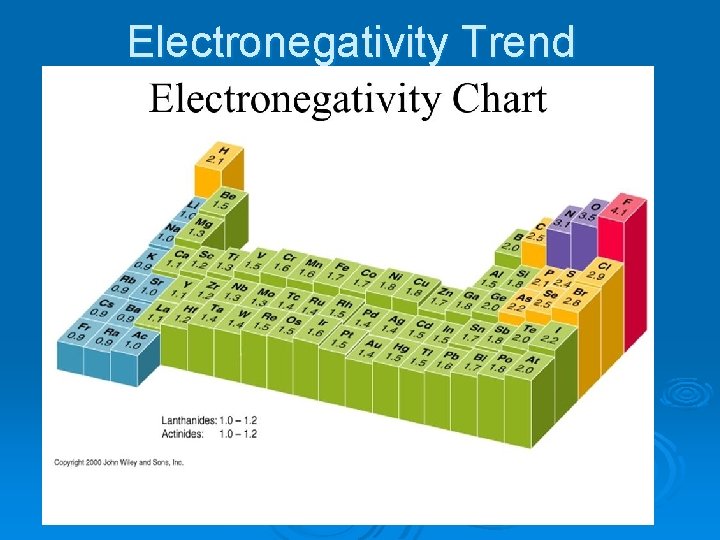
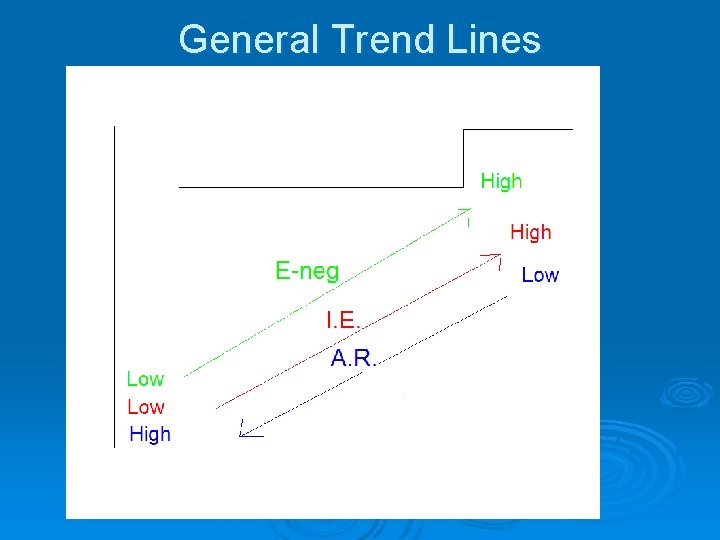
Electronegativity Ø The ability of an atom to attract electrons away from a different atom. Ø (How strongly an atom pulls on electrons from other atoms) Ø Most electronegative: Fluorine. Ø Least electronegative: Alkali Metals Ø The noble gases have minimal electronegativity.

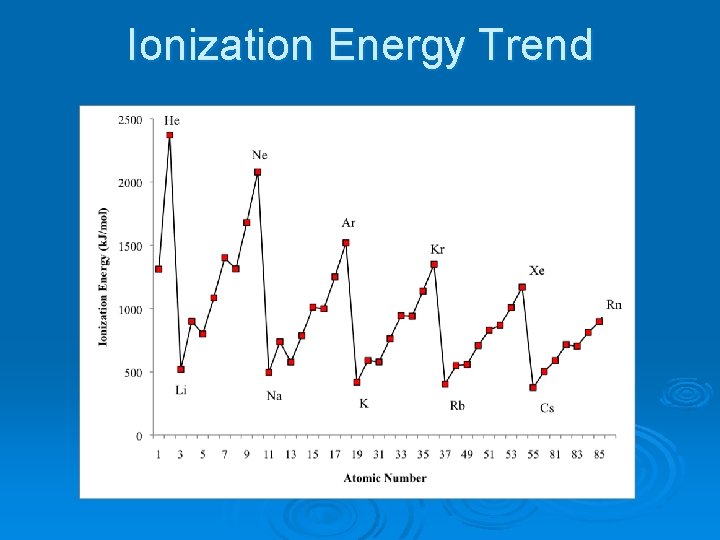
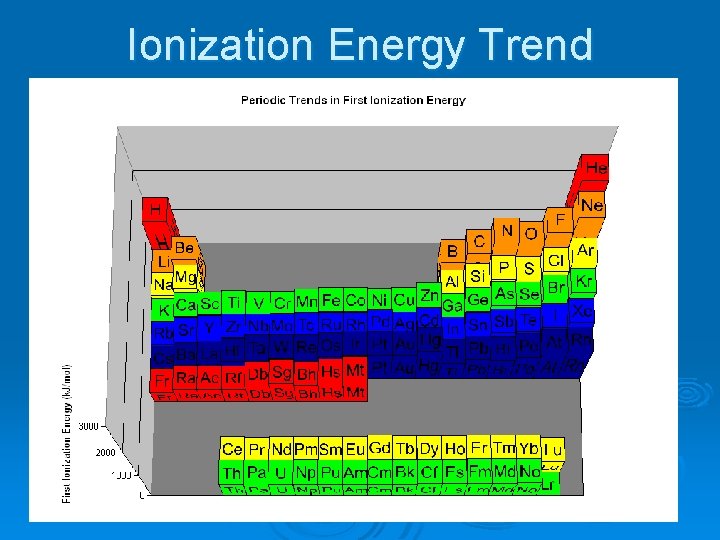
Ionization Energy Ø The amount of energy required to remove the most loosely held electron from an atom. Ø (How strongly an atom holds on to its own electrons) Ø Highest I. E: Helium and Noble Gases Ø Lowest I. E: Alkali Metals

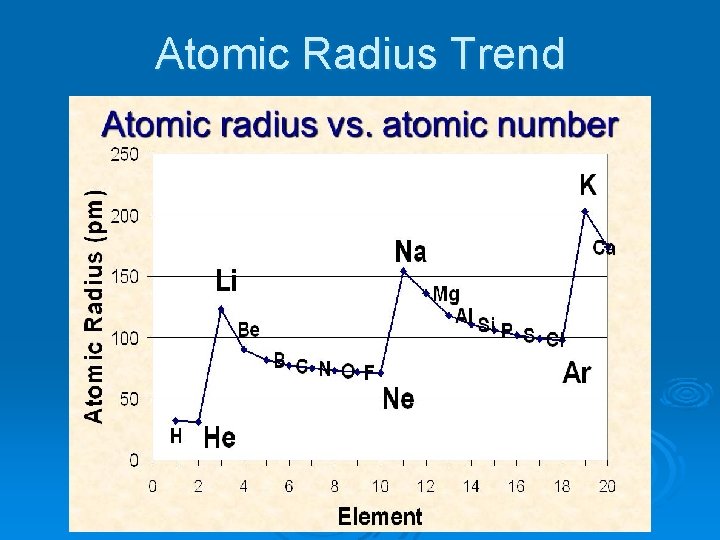
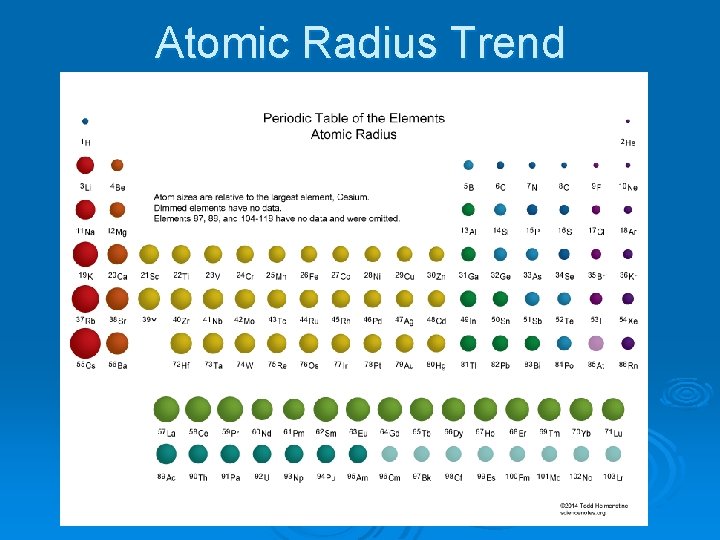
Atomic Radius Ø The distance from the center of the nucleus of an atom to the edge of the electron cloud. Ø (How big an atom is) Ø Largest: Francium Ø Smallest: Hydrogen and Helium

Electronegativity Trend

Electronegativity Trend

Ionization Energy Trend

Ionization Energy Trend

Atomic Radius Trend

Atomic Radius Trend

General Trend Lines

***WHY do these trends exist? *** Ø Across a Period – As we move from Left Right across a period, the atoms have more protons but the same number of electron energy levels. l l The extra protons pull more strongly on electrons (E-neg and I. E. increase) The extra protons pull the electron cloud closer (A. R. gets smaller) Ø **Increased Positive Charge in the nucleus = Stronger Pull on Electrons**

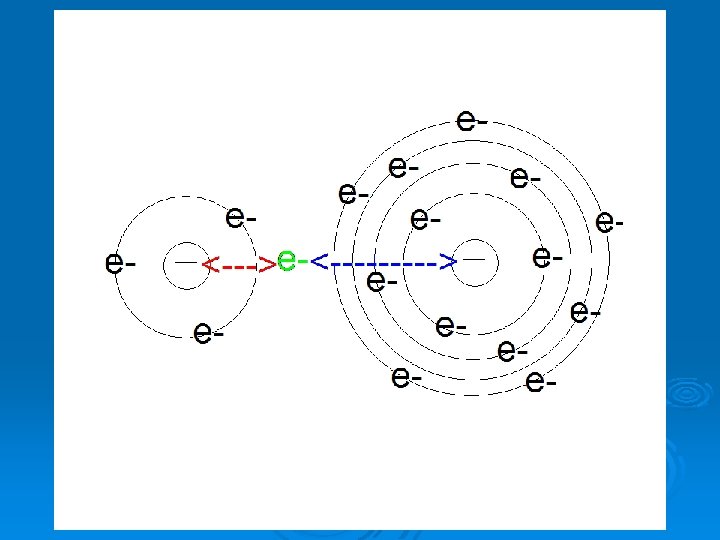
***WHY do these trends exist? *** Ø Down A Group – As we move down a group, every atom has an extra energy level of electrons. Ø The INNER electrons shield (block) the positive charge of the nucleus and push the valence level further away. l l Making it more difficult for the nucleus to attract electrons (E-neg and I. E. decrease) Allowing the electron cloud to expand outwards (A. R. gets bigger) Ø Electron Shielding **Decreased Effective Nuclear Charge**


Atomic Radius of Ions Ø Positive Ions (+) l Fewer e-, smaller e- cloud, + nucleus pulls cloud closer, atomic radius decreases (especially when valence level is vacated!!) Ø Negative Ions (-) l More e-, bigger e- cloud, cloud expands further out, atomic radius increases