Periodic Trends Part I Atomic Radius Periodic Law












- Slides: 22

Periodic Trends Part I: Atomic Radius

Periodic Law When the elements are arranged in order of increasing atomic number, there is a regular and repeating pattern in their physical and chemical properties

How could we measure the diameter of cotton candy?

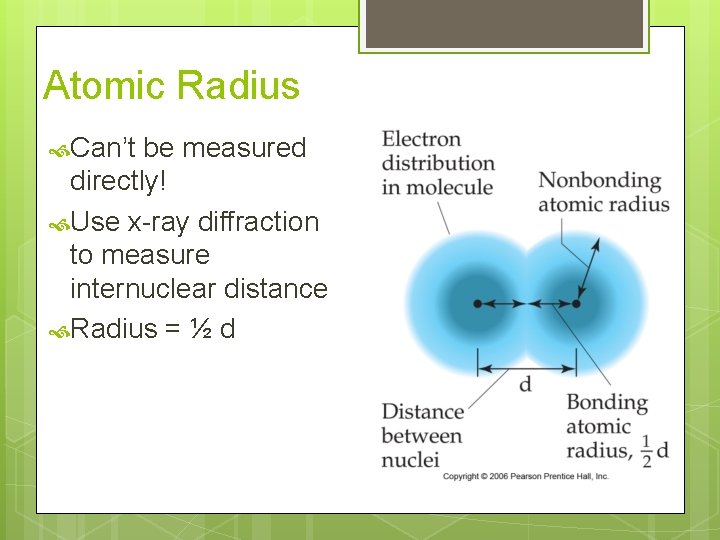
Atomic Radius Can’t be measured directly! Use x-ray diffraction to measure internuclear distance Radius = ½ d

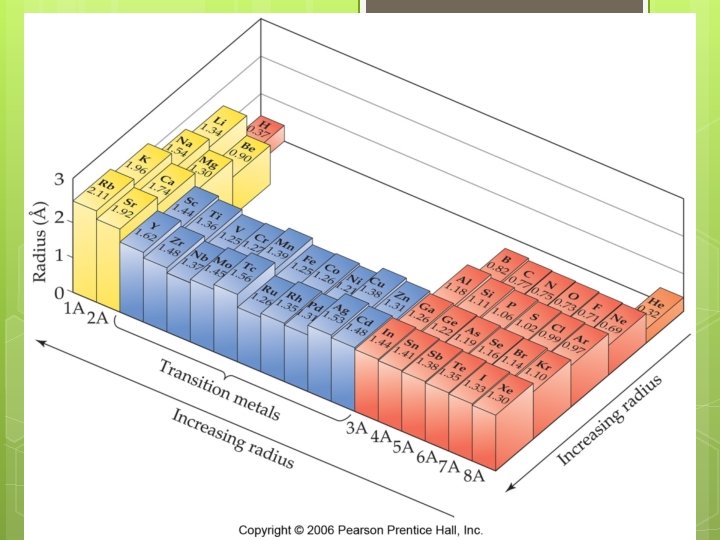
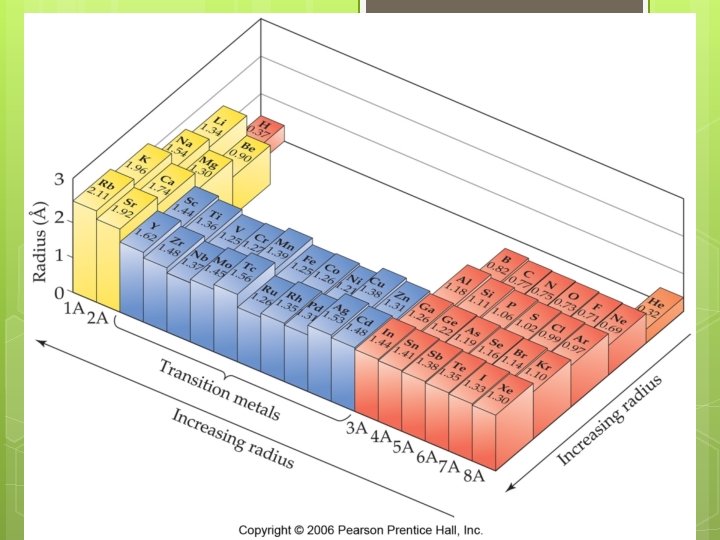
Atomic Radius Trends

Explaining the atomic radius trend Atom radius decreases from left to right

Explaining the atomic radius trend Atom radius decreases from left to right Because tightly the outermost electrons are held more

Explaining the atomic radius trend Atomic radius decreases from left to right… because the outermost electrons are held more tightly Nuclear charge (# of protons) increases left to right STRONGER COULOMBIC ATTRACTION electron-electron repulsions are essentially constant Keep filling the same energy level

Atomic Radius Trends

Explaining the atomic radius trend Atomic radius increases down a column

Explaining the atomic radius trend Atomic radius increases down a column Valence electrons are placed in higher energy levels farther from the nucleus Outermost WEAKER More electrons are not held tightly COULOMBIC ATTRACTION core electrons more electron-electron repulsions (i. e. , more shielding)

Practice Problems Handout

Ions Cations Anions

Atoms will gain or lose electrons… To have an electron configuration that is isoelectronic with a noble gas

Atoms will gain or lose electrons… To have an electron configuration that is isoelectronic with a noble gas • Completely filled sublevels • 8 valence electrons OCTET RULE

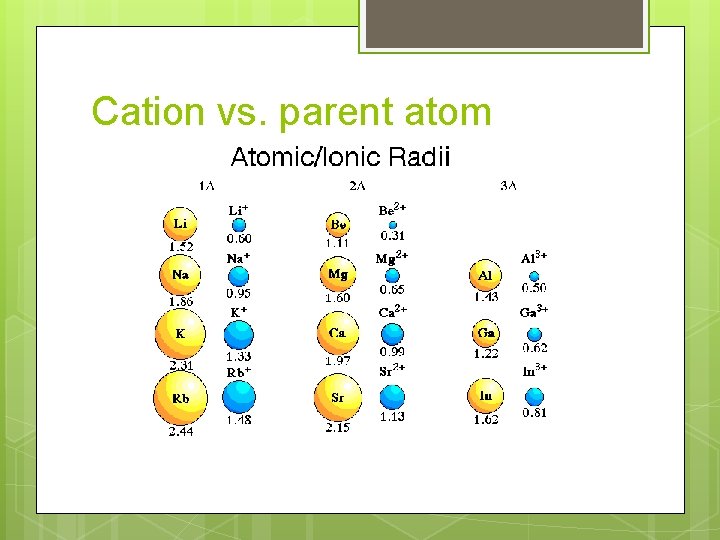
Cation vs. parent atom

Cation vs. parent atom Cations typically have a SMALLER radius than the parent atom Why? Fewer electrons Fewer electron-electron repulsions Fewer occupied energy levels

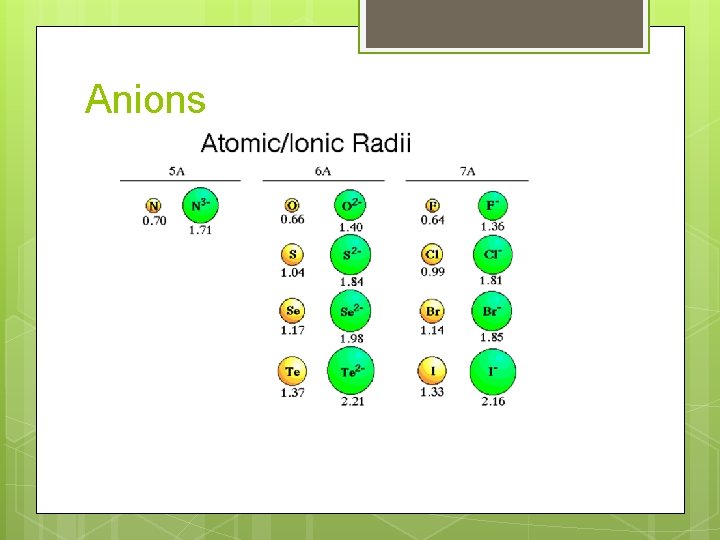
Anions

Anions vs. parent atom Anions typically have LARGER radii than the parent atoms Why? More electron-electron repulsions

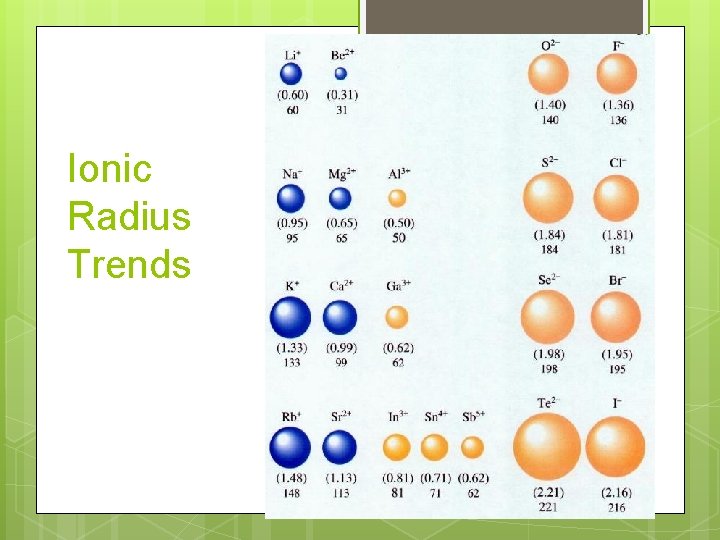
Ionic Radius Trends

Summary of ionic radius trends Group trend Period trend

Which of the following would have the larger radius? Ca P or Ca 2+ or P 3 - Ca 2+ or Cl- K+ or Sc 3+ Cl- or I- P 3 - or S 2 -