Periodic Trends in Properties on the Periodic Table

Periodic Trends in Properties on the Periodic Table

The Plan Essential Question: What causes the trends of the properties in the periodic table? l Discuss Some Properties of Atoms l Explore the Trends l Try to Explain the Trends

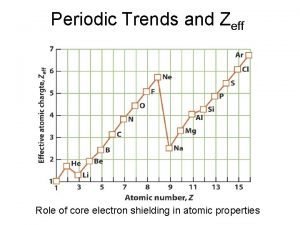
Effective Nuclear Charge - (Zeff) – the charge an electron “feels” from the nucleus l Zeff = Z – S, where Z is the atomic number and S is the shielding experienced by the outer electrons l The electrons in the outer energy levels don’t feel the full charge of the nucleus because the core electrons help shield them from the nucleus. l
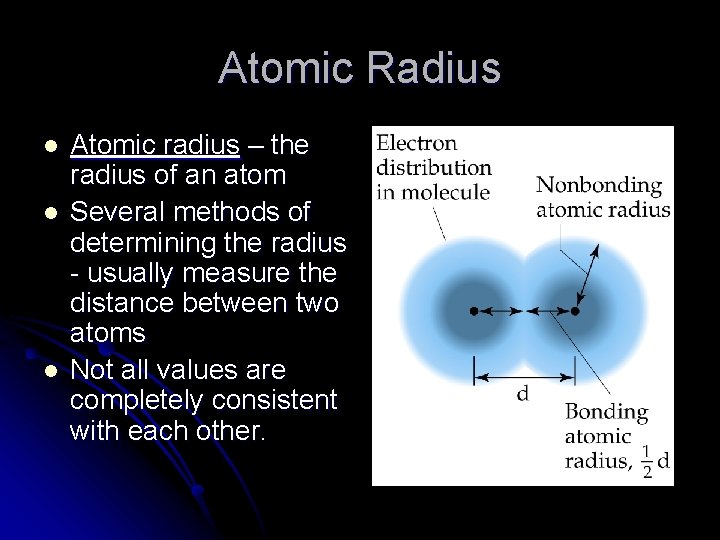
Atomic Radius l l l Atomic radius – the radius of an atom Several methods of determining the radius - usually measure the distance between two atoms Not all values are completely consistent with each other.

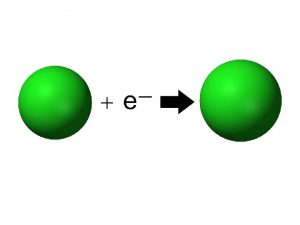
Ionization Energy l Ionization Energy – the energy required to remove one electron from an atom l Essentially this is the process of making a positive ion. X X+ + e -

Electronegativity l Electronegativity – the ability of an atom in a compound to attract electrons to itself l Electrons in compounds spend the most time around atoms with high electronegativities. l Elements with high electronegativities have partial negative charges in compounds.

Periodic Trends Lab In your lab groups, predict what trends you think might occur down a family and across a period for each of the properties we discussed: Effective nuclear charge Atomic radius Ionization energy Electronegativity Record on the index card for discussion later

Effective Nuclear Charge l As you travel left to right across a period: l Atomic number increases l Shielding stays roughly the same l Therefore Zeff increases significantly across a period l As you travel down a family l Atomic number increases sharply l Shielding increases sharply l Zeff does increase down a family but not as much as you might expect.

Atomic Radius l As you travel left to right across a period: l Effective nuclear charge increases l Electrons are being added to the same n shell l n values have somewhat to do with distance from the nucleus. l Size of atoms shrink as you move to the right. l As you travel down a family l Effective nuclear charge increases more slowly l Electrons are added to n shells farther away l Size of atoms increase down the family

Ionization Energy l As you travel left to right across a period l Effective nuclear charge increases l Harder to remove an electron therefore… l Ionization Energy increases l As you travel down a family l Effective nuclear charge increases slowly l Outer electrons are farther away from the nucleus l Ionization Energy decreases

Ionization Energy

Ionization Energy

Electronegativity l As you travel left to right across a period: l Effective nuclear charge increases strongly l Electronegativity increases l As you travel down a family l Effective nuclear charge increases slowly l Outer electrons are being added in shells farther and farther away l Electronegativity decreases
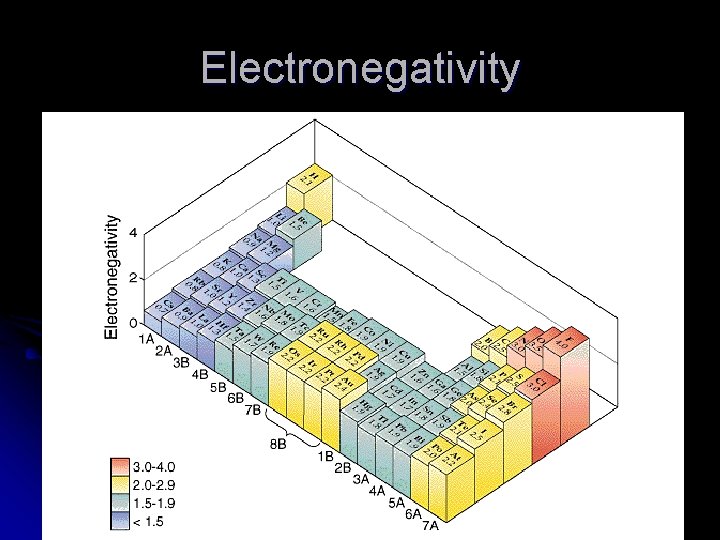
Electronegativity
Ionic Radius l Ionic Radius – size of an ion l Consider two different relationships l Relationship between atom and ion l Relationship between ions and atoms with the same number of electrons l “isoelectronic series”
Ionic Radius

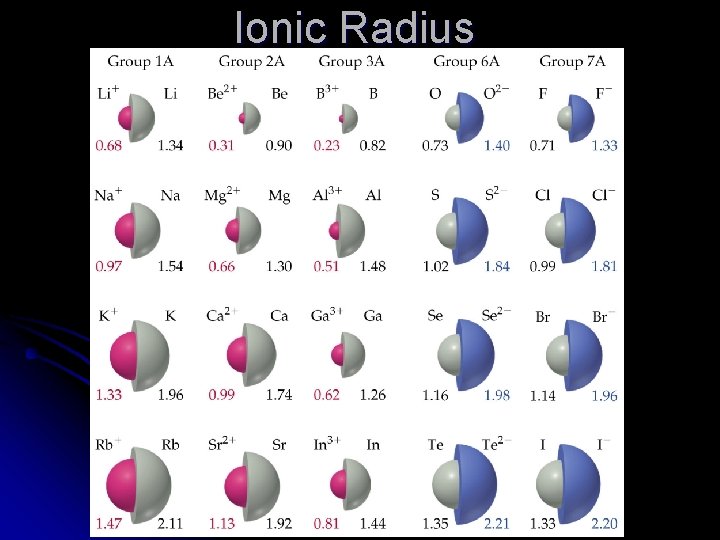
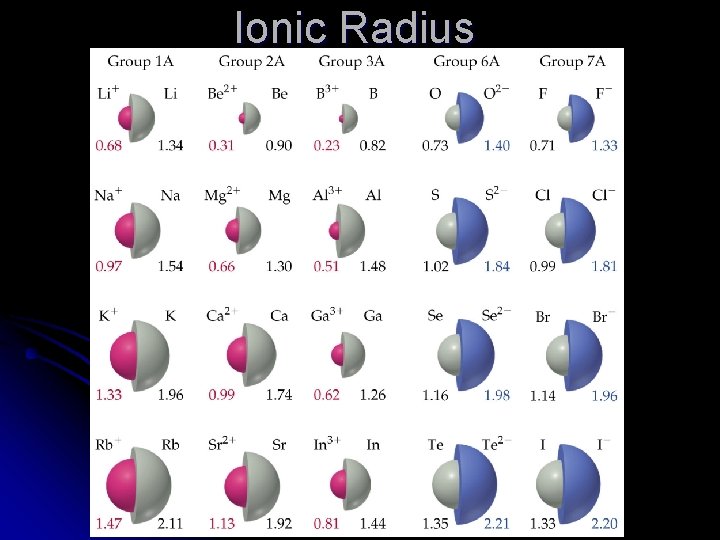
Ionic vs. Atomic Number of protons always stays the same l For negative ions (anions) l l Electrons are added to the same outer shell l Electron-electron repulsions increase l Anions are larger than their atoms l For positive ions (cations) l Electrons are removed to leave one less shell l n shells describe distance from nucleus l Cations are smaller than their atoms
Ionic Radius

Isoelectronic series All have the same number of electrons l Number of protons changes l Therefore effective nuclear charge increases l Electrons are pulled in tighter l The negative ions are larger than the positive ions with the same number of electrons. l

Practice Problems l Arrange the following elements in order of decreasing atomic radius: l As, l O, Sn, Ge, Ne, Ba, He Arrange the following elements in order of increasing electronegativity l Al, Mg, P, Sr, O, F, Rb

Ticket Out the Door l Arrange the following elements in order of increasing ionization energy: l Cs, Ba, Y, In, Ga, Si, P, F

Test Review Problem Challenge Problem l Place the following in order of increasing size: Ne, Cl-, Ar, Na+ l
- Slides: 22