Periodic Trends Atomic Radii Elemental Properties and Patterns















- Slides: 23

Periodic Trends: Atomic Radii Elemental Properties and Patterns

The Periodic Law Dimitri Mendeleev (1869/1871) was the first scientist to publish an organized periodic table of the known elements. He was taking a chemistry course in Russia and tried to find a way to organize the periodic table.

The Periodic Law Mendeleev even went out on a limb and predicted the properties of 2 at the time undiscovered elements. He was very accurate in his predictions, which led the world to accept his ideas about periodicity and a logical periodic table.

The Periodic Law Mendeleev understood the ‘Periodic Law’ which states: When arranged by increasing atomic number, the chemical elements display a regular and repeating pattern of chemical and physical properties.

The Periodic Law Atoms with similar chemical properties and behavior appear in groups or families (vertical columns named by Roman numerals with A or B) on the periodic table. They are similar because they all have the same number of valence (outer shell) electrons, which governs their chemical behavior. Periods– horizontal rows on periodic table

Periodic Trends There are several important atomic characteristics that show predictable trends that you should know. Atomic properties— Deal with only single atoms

Electron Shielding The full, positive nuclear charge is “SHIELDED” from outer shell electrons by all the other electrons between them Electrons closer to the nucleus shield other electrons further away. Full attractive force is not felt by outer electrons Electrons have different energies and therefore, located at different regions surrounding the nucleus Based on electrostatic field + positively charged nucleus

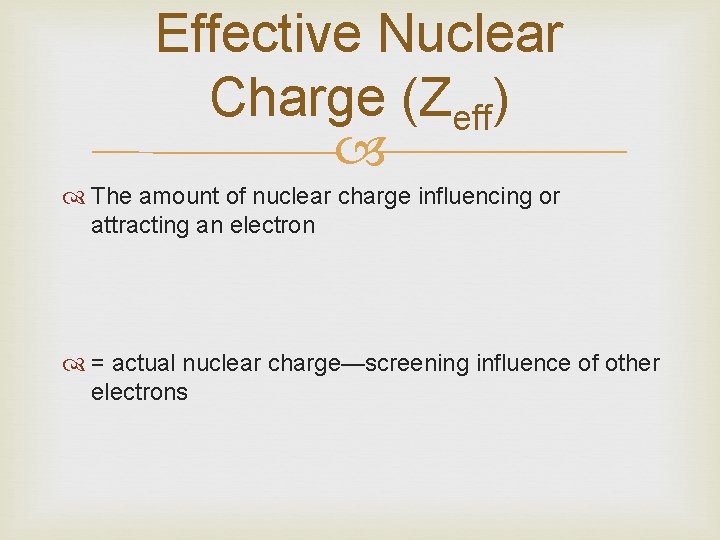
Effective Nuclear Charge (Zeff) The amount of nuclear charge influencing or attracting an electron = actual nuclear charge—screening influence of other electrons

Atomic Radii An atom’s size affects chemical and physical properties of an atom Atom’s size is related to the radius of the atom Radius of atom = ½ distance between nuclei of 2 adjacent atoms of the same element Radius is the distance from the center of the nucleus to the “edge” of the electron cloud. half the distance between the nuclei of 2 bonded atoms.

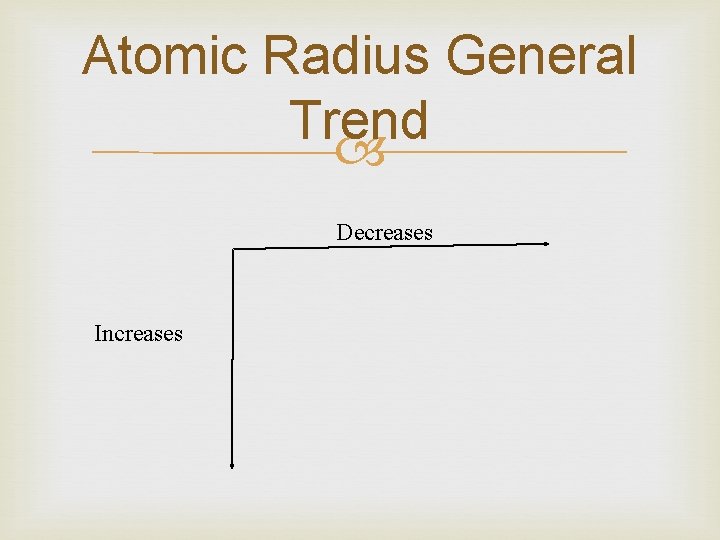
Atomic Radius General Trend Decreases Increases

Atomic Radius The effect is that the more positive nucleus has a greater pull on the electron cloud. The nucleus is more positive and the electron cloud is more negative. The increased attraction pulls the cloud in, making atoms smaller as we move from left to right across a period—MOVING ACROSS TABLE More and more electrons are added and placed at higher and higher energy levels so atomic size increases— MOVING DOWN TABLE

1. Metallic Radii Half the distance between nuclei of adjacent atoms in a metal Radius of metallic atoms > 75% of elements are metals

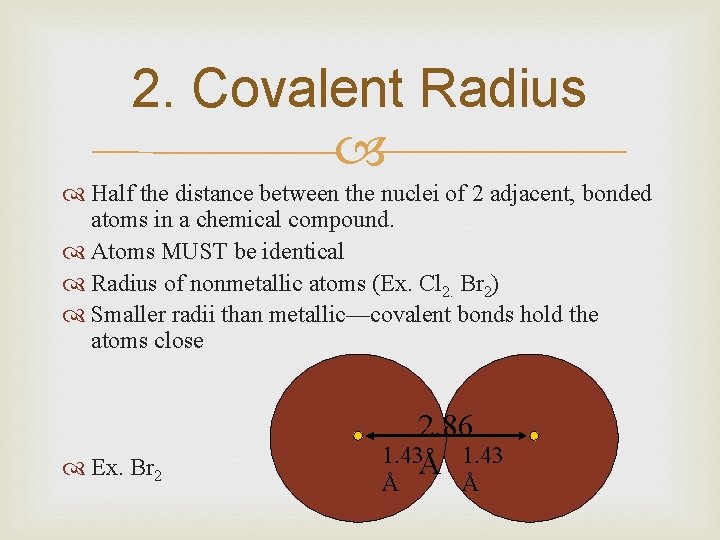
2. Covalent Radius Half the distance between the nuclei of 2 adjacent, bonded atoms in a chemical compound. Atoms MUST be identical Radius of nonmetallic atoms (Ex. Cl 2. Br 2) Smaller radii than metallic—covalent bonds hold the atoms close Ex. Br 2 2. 86 1. 43Å 1. 43 Å Å

Ionic Radii Ions Atoms that have gained or lost electrons (anion, cation) Electrons gained/lost from valence shell (outer electron shell) Defined by the distance between the nuclei of 2 ions Studies with crystal structures Radii related to original atomic radii

Ionic Radii (cont. ) 1) Anions LARGER size than original neutral atom Generally nonmetals Additional electrons cause the atom’s size to increase Electrons repel as increase in number, causes size to increase

Ionic Radii (cont. ) 2) Cations SMALLER size than original neutral atom Generally metals Removal of electrons causes the atom’s size to decrease more attractive force from nucleus, nucleus can pull remaining electrons towards it.

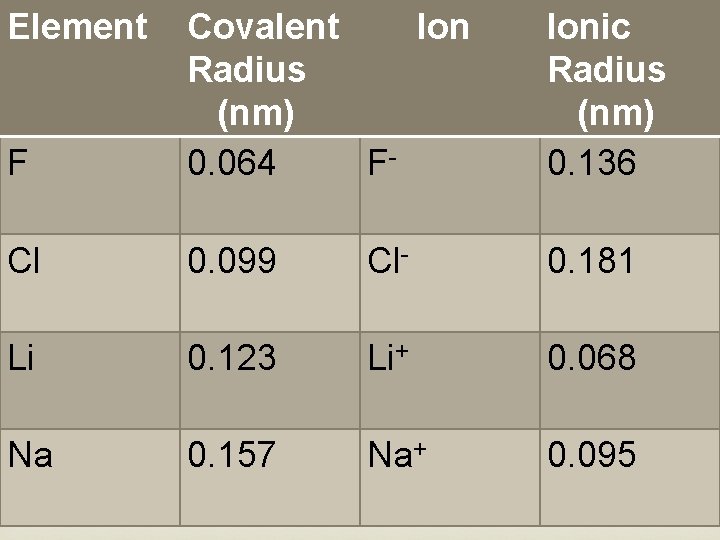
Element F Covalent Ion Radius (nm) F 0. 064 Ionic Radius (nm) 0. 136 Cl 0. 099 Cl- 0. 181 Li 0. 123 Li+ 0. 068 Na 0. 157 Na+ 0. 095

Example 1 Compare atomic sizes of Na and Cl Neutral Na > Cl Ion Na+ < Cl-

Isoelectric Applies to either atoms or ions Atoms or ions with the SAME number of electrons So electron configuration is the same as well Does not state the protons stay the same As the proton number increases but the electrons stay the same, the positive nucleus holds electrons more tightly

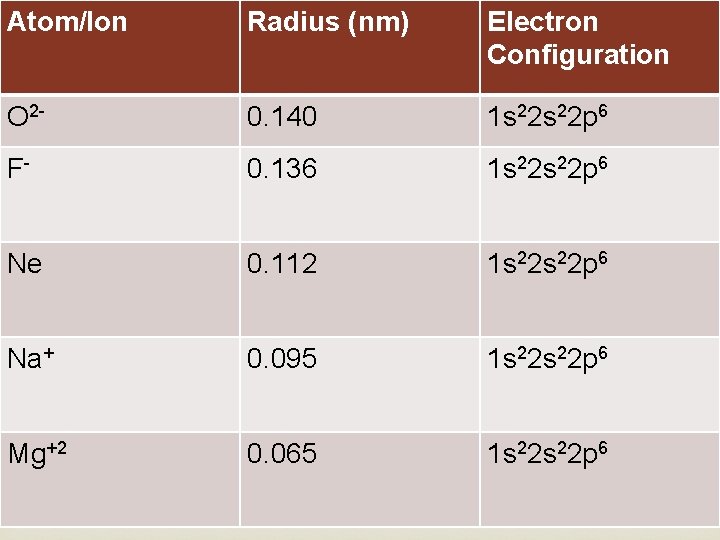
Atom/Ion Radius (nm) Electron Configuration O 2 - 0. 140 1 s 22 p 6 F- 0. 136 Ne 0. 112 1 s 22 p 6 Na+ 0. 095 1 s 22 p 6 Mg+2 0. 065 1 s 22 s 22 p 6

Example 2: Which of the following ions has the LARGEST radius? A) Na+ B) Mg+2 C) S 2 D) Cl. E) Se 2 -

Example 3: Which of the following ions has the SMALLEST radius? Why? A) K+ B) Li+ C) Be+2 D) O 2 E) F-

Example 4: Which of the following isoelectric ions is the LARGEST? Why? A) Mn+7 B) P 3 C) S 2 D) Sc 3+ E) Ti 4+