Periodic Trends Activity 3 ELECTRONS EXAM CORRECTIONS PERIODICITY














- Slides: 17

Periodic Trends Activity 3 ELECTRONS EXAM CORRECTIONS PERIODICITY PRACTICE

Objectives Today I will be able to: Explain why I got multiple choice questions wrong on the electrons exam Analyze how the trends of atomic radius, ionization energy and electronegativity change across a period and down a family Compare the atomic radius, ionization energy and electronegativity of 2 or more elements Informal assessment: monitoring student questions and interactions as we complete the practice and exam corrections Formal assessment: analyzing the students interpretations of the trends, practice and exit ticket Common Core Connection Model with mathematics

Lesson Sequence Evaluate: Warm – Up Explain: Electrons Exam Corrections Elaborate: Periodicity Practice Worksheet Elaborate: Applying the trends worksheet Evaluate: Exit Ticket

Warm - Up How does the atomic radius change across a period and down a family? How does electronegativity/ionization energy change across a period and down a family?

Objective Today I will be able to: Explain why I got multiple choice questions wrong on the electrons exam Analyze how the trends of atomic radius, ionization energy and electronegativity change across a period and down a family Compare the atomic radius, ionization energy and electronegativity of 2 or more elements

Homework Finish Analyzing the Trends Worksheet Study for the Multiple Choice Re-quiz at the beginning of next class

Agenda Warm-Up Electrons Exam Corrections Periodicity Practice Analyzing the trends Worksheet Exit Ticket

Complete the corrections at your desk. Ms. Ose will explain the requirements for the re-quiz next class ELECTRONS EXAM CORRECTIONS

WHY DO THE TRENDS OCCUR?

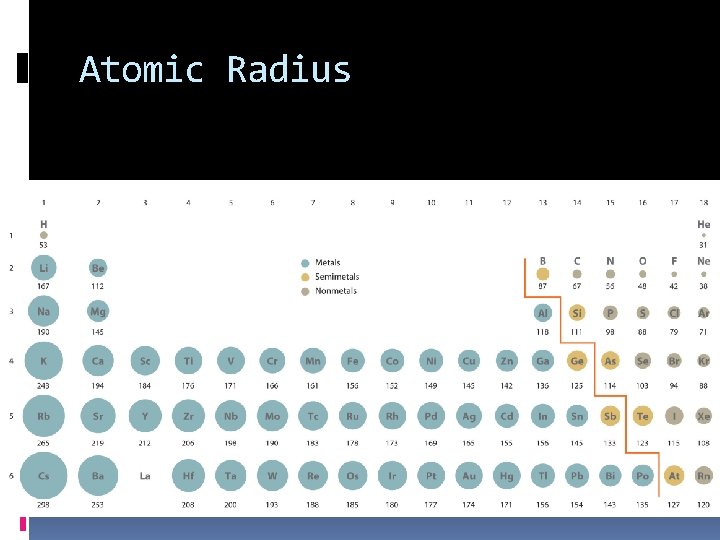
Atomic Radius… Decreases across a period Why? More protons in the nucleus, pulling the electron cloud closer to the nucleus Increases down a family Why? More energy levels for the electrons to fill

Atomic Radius

Ionization Energy Increases across a period Why? A greater number of valence electrons makes it more difficult to remove an electron Decreases down a family Why? The electrons are farther away from the nucleus. They are less attracted to the atom making them easier to remove

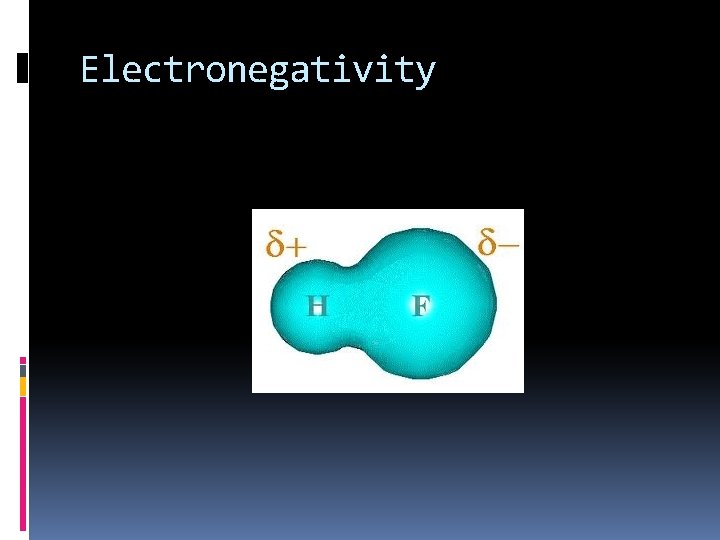
Electronegativity Increases across a period Why? Atoms are closer to reaching stability so they want to attract electrons Decreases down a family Why? The electrons are farther away from the nucleus in higher energy levels. They are not attracted to the nucleus as easily

Electronegativity

Complete the worksheet using your notes. Ask Ms. Ose for help if you have questions we will review selected problems. PERIODICITY PRACTICE WORKSHEET

Complete the analyzing the trends worksheet. If you do not finish in class, it will become homework. Be sure to respond to all parts of the question ANALYZING THE TRENDS WORKSHEET

Exit Ticket For the following set of elements: Ba Mg O Circle the element that has the smallest atomic radius Underline the element with the smallest ionization energy Box the element with the greatest electronegativity value
 Periodic table with electronegativity and ionization energy
Periodic table with electronegativity and ionization energy 15/999 mass street periodic table, o 8
15/999 mass street periodic table, o 8 Periodic trends activity worksheet
Periodic trends activity worksheet Debye huckel equation
Debye huckel equation What is periodicity?
What is periodicity? First dental home visit documentation form
First dental home visit documentation form Feeding periodicity adalah
Feeding periodicity adalah Chemsheets periodicity
Chemsheets periodicity C n o f electron affinity
C n o f electron affinity 42 electron configuration
42 electron configuration Ap chemistry atomic structure and periodicity
Ap chemistry atomic structure and periodicity Bright futures screening guidelines
Bright futures screening guidelines Oxygen periodic trends
Oxygen periodic trends Electronegativity and atom size
Electronegativity and atom size Wuchereria bancrofti periodicity
Wuchereria bancrofti periodicity Chapter 7 atomic structure and periodicity
Chapter 7 atomic structure and periodicity Valence electrons
Valence electrons Group 1 valence electrons
Group 1 valence electrons