PERIODIC TRENDS A periodic trend is a repeating






















- Slides: 33

PERIODIC TRENDS

A periodic trend is a repeating pattern that occurs due to the position of electrons in an atom.

Three factors: 1)Effective Nuclear Charge: The attraction or “pull” on valence electrons by protons in the nucleus.

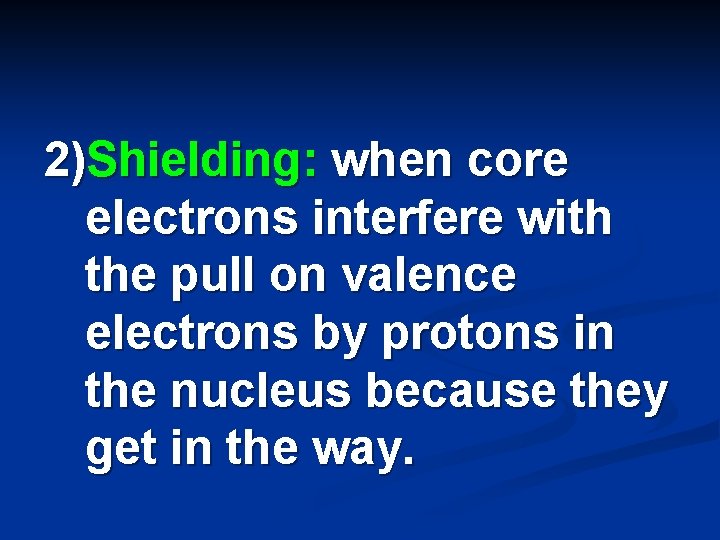
2)Shielding: when core electrons interfere with the pull on valence electrons by protons in the nucleus because they get in the way.

3)Electron Repulsion: electrons repel each other because they are all negatively charged.

Why do the trends occur? n. As you move ACROSS a period, valence electrons are added to the same P. E. L.


n Effective nuclear charge increases because you are adding more e- (and p+), but at the same distance from nucleus, so p+ “pull” on valence e- is greater.

n Shielding is not affected (no increase in the number of core electrons)

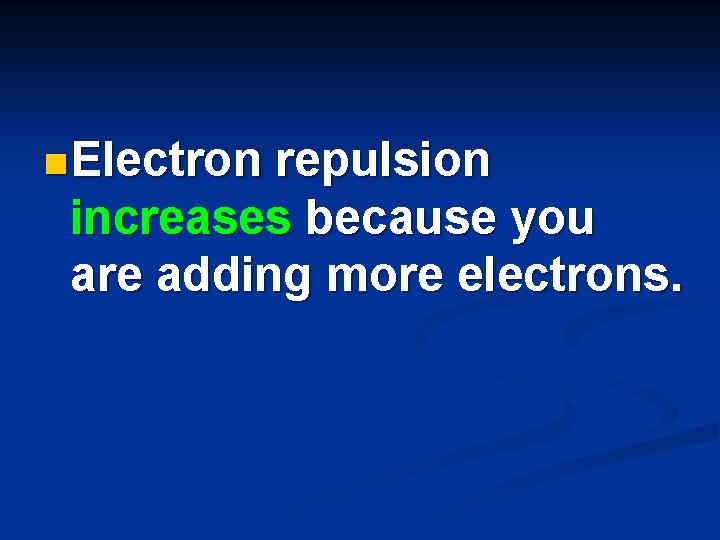
n Electron repulsion increases because you are adding more electrons.

Why do the trends occur? n. As you move DOWN a group, valence electrons are added to different P. E. L.


n Effective Nuclear charge decreases because even as you are adding more e- (and p+), they are further from the nucleus, so the “pull” on valence e- by p+ is less effective.

n Shielding increases because as e- are added at P. E. L. further from the nucleus, there are more core e- to interfere.

n. Electron repulsion increases because you are adding more electrons.

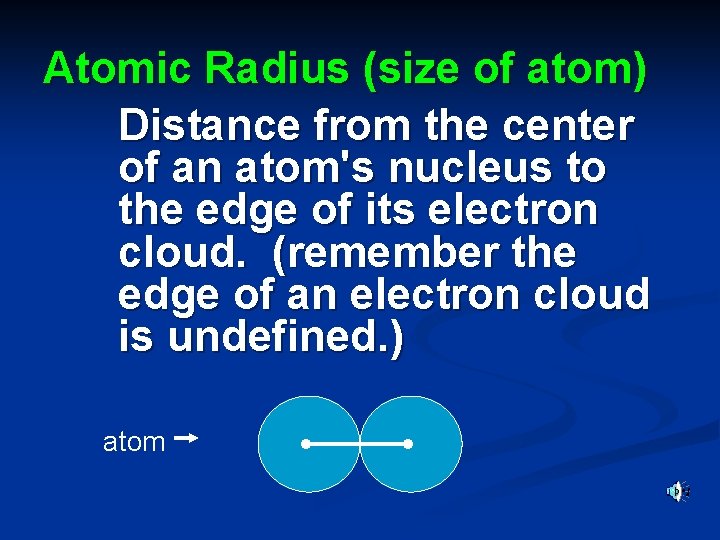
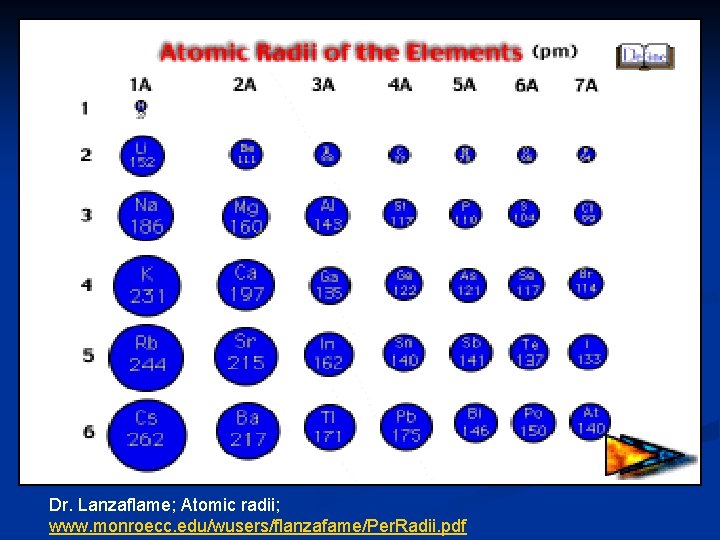
Atomic Radius (size of atom) Distance from the center of an atom's nucleus to the edge of its electron cloud. (remember the edge of an electron cloud is undefined. ) atom

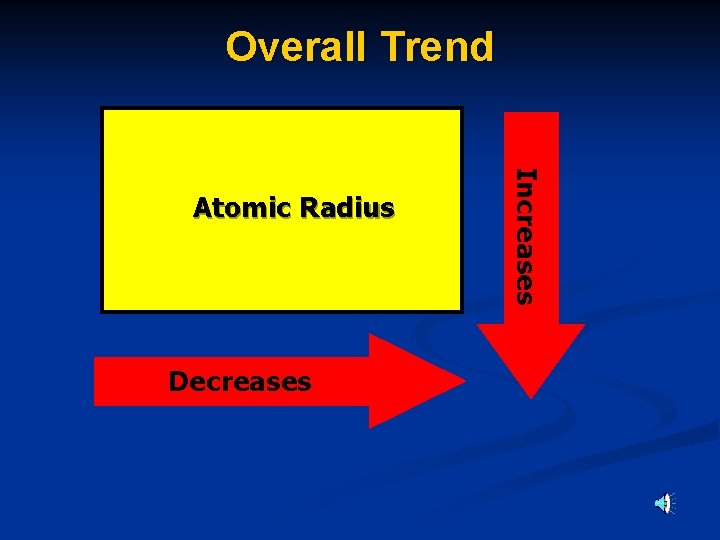
Atomic radius decreases as you move ACROSS the periodic table. Atomic radius increases as you move DOWN the periodic table.

Overall Trend Decreases Increases Atomic Radius

Dr. Lanzaflame; Atomic radii; www. monroecc. edu/wusers/flanzafame/Per. Radii. pdf

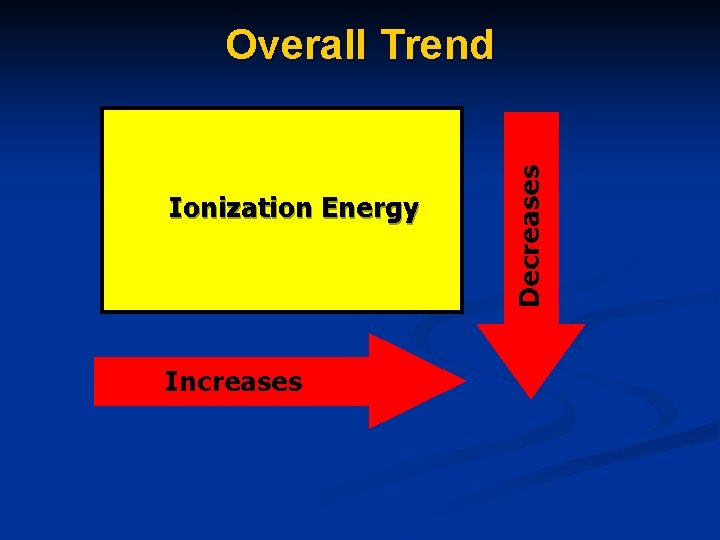
Ionization Energy The amount of energy needed to remove one valence electron from a gaseous atom of an element.

Ionization Energy increases as you move ACROSS the periodic table. Ionization Energy decreases as you move DOWN the periodic table.

Ionization Energy Increases Decreases Overall Trend

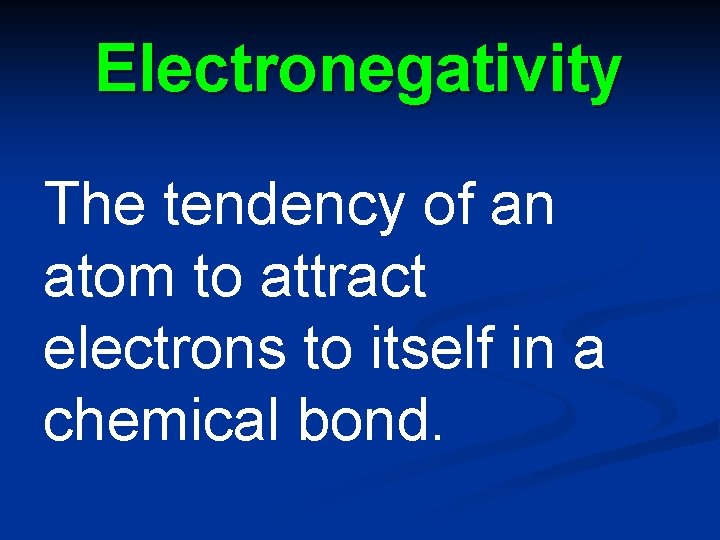
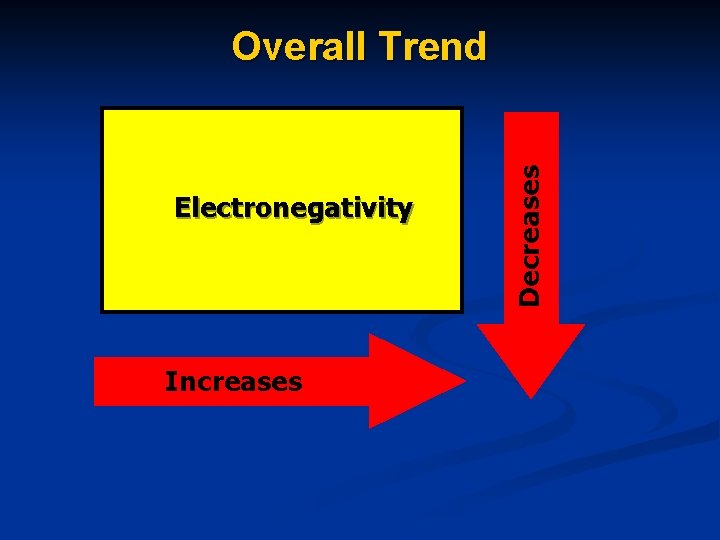
Electronegativity The tendency of an atom to attract electrons to itself in a chemical bond.

Electronegativity increases as you move ACROSS the periodic table. Electronegativity decreases as you move DOWN the periodic table.

Electronegativity Increases Decreases Overall Trend

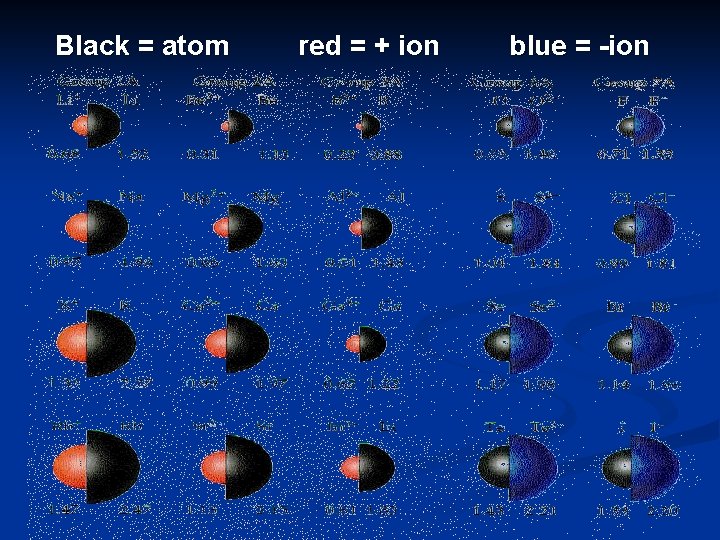
Ionic Radius (size of ion) n. An ion is an atom that has lost or gained valence electron(s).

n Positive ions (cations) are formed when an atom loses one or more valence electrons n Negative ions (anions) are formed when an atom gains one or more valence electrons.

Trend A positive ion is smaller than its neutral atom. A negative ion is larger than its neutral atom.


Black = atom red = + ion blue = -ion

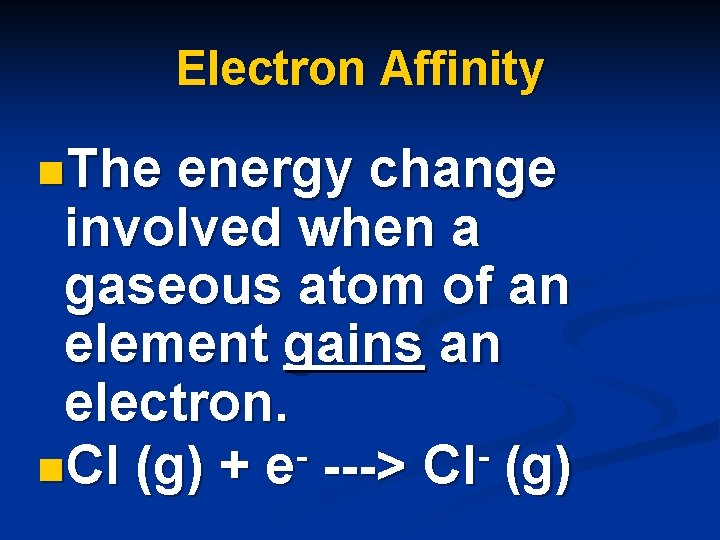
Electron Affinity n. The energy change involved when a gaseous atom of an element gains an electron. n. Cl (g) + e ---> Cl (g)

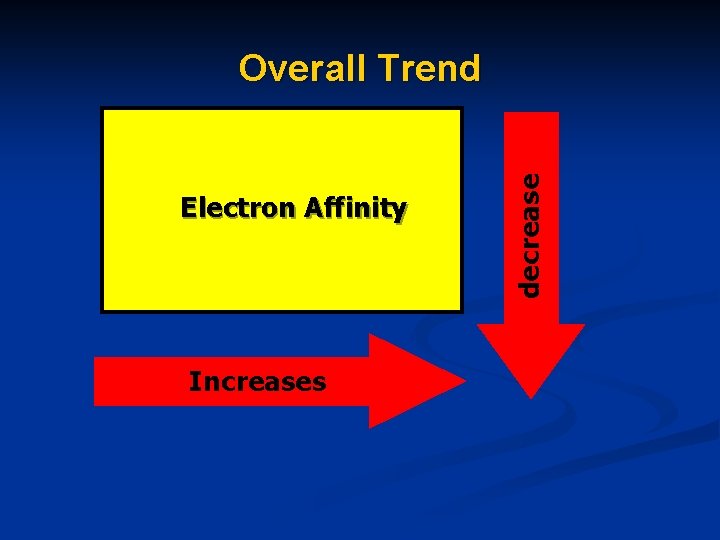
Electron Affinity Increases decrease Overall Trend

n A negative electron affinity value indicates n A positive electron affinity value indicates