Periodic Table Trends Atomic Radius The radius of

Periodic Table: Trends
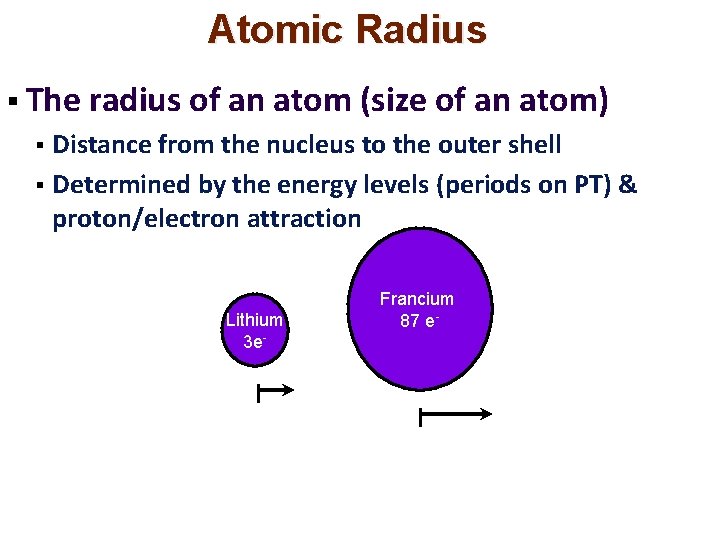
Atomic Radius § The radius of an atom (size of an atom) Distance from the nucleus to the outer shell § Determined by the energy levels (periods on PT) & proton/electron attraction § Lithium 3 e- Francium 87 e-
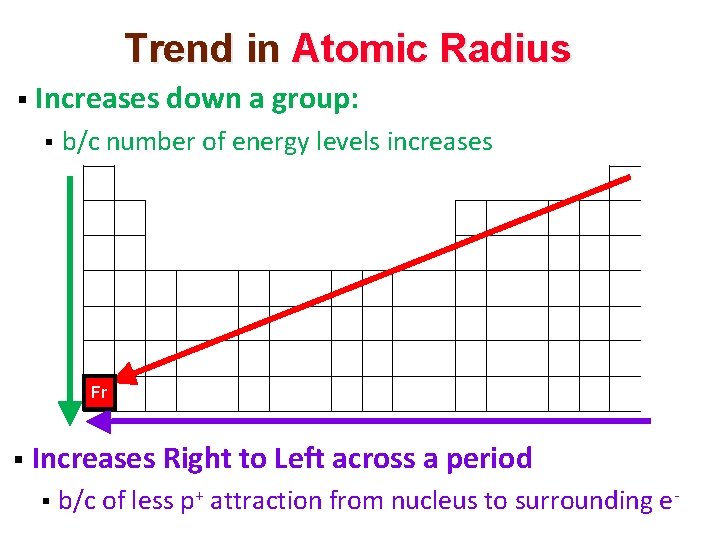
Trend in Atomic Radius § Increases down a group: § b/c number of energy levels increases Fr § Increases Right to Left across a period § b/c of less p+ attraction from nucleus to surrounding e-
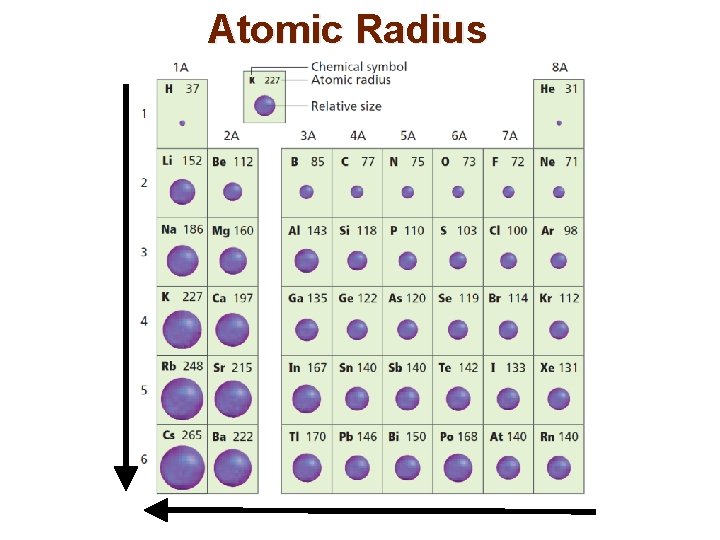
Atomic Radius
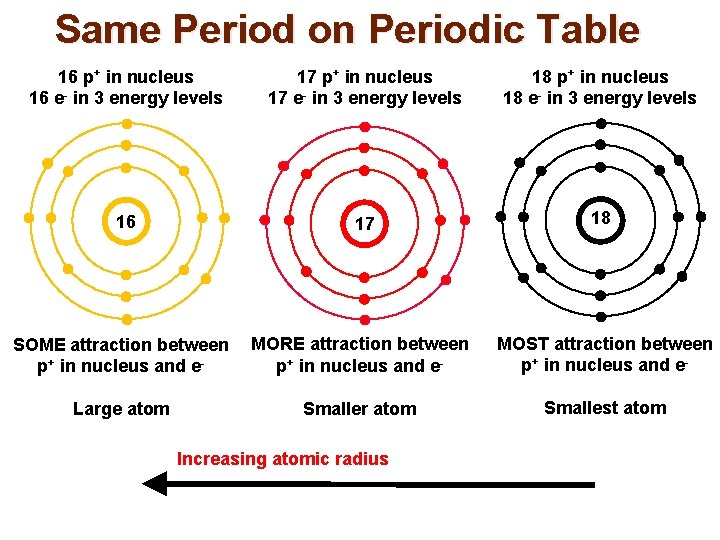
Same Period on Periodic Table 16 p+ in nucleus 16 e- in 3 energy levels 17 p+ in nucleus 17 e- in 3 energy levels 18 p+ in nucleus 18 e- in 3 energy levels 16 17 18 SOME attraction between p+ in nucleus and e- MORE attraction between p+ in nucleus and e- MOST attraction between p+ in nucleus and e- Large atom Smaller atom Smallest atom Increasing atomic radius
Atomic Radius Examples § Which element is larger? Explain. Silicon or Sulfur Silicon’s p+ don’t attract the e- as close as Sulfur’s p+ do § Which element is smaller? Explain. Barium or Zirconium Zr has 5 energy levels, Ba has 6, so Zr is smaller
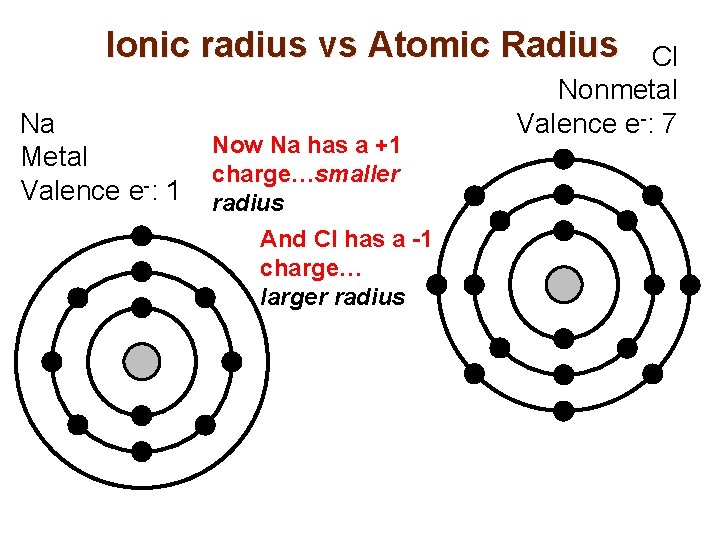
Ionic radius vs Atomic Radius Na Metal Valence e-: 1 Now Na has a +1 charge…smaller radius And Cl has a -1 charge… larger radius Cl Nonmetal Valence e-: 7
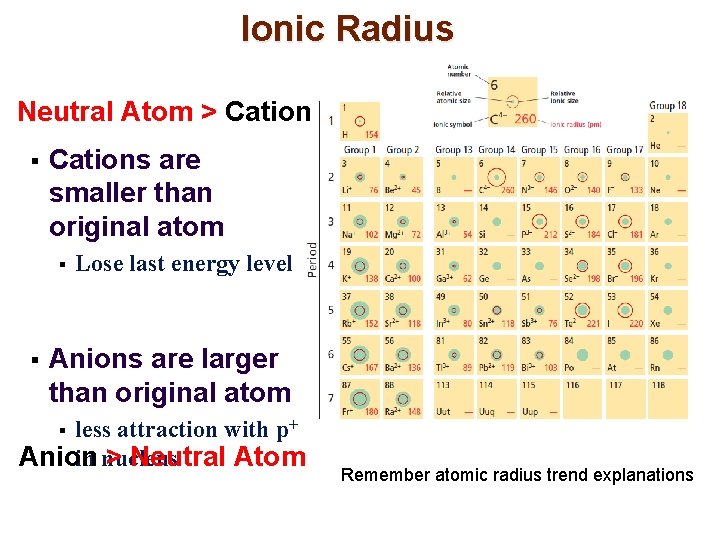
Ionic Radius Neutral Atom > Cation § Cations are smaller than original atom § § Lose last energy level Anions are larger than original atom less attraction with p+ Anion > Neutral Atom in nucleus § Remember atomic radius trend explanations
Ionic Radius Examples § Which is larger in size: Ca+2 or Ca ? Cations are smaller than neutral atoms § Which is larger in size: Cl-1 or Cl ? Anions are larger than neutral atoms
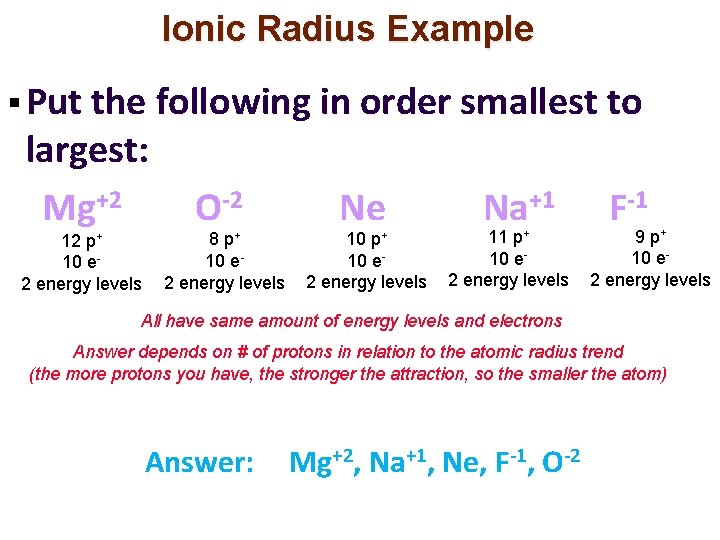
Ionic Radius Example § Put the following in order smallest to largest: Mg+2 12 p+ 10 e 2 energy levels O-2 8 p+ 10 e 2 energy levels Ne 10 p+ 10 e 2 energy levels Na+1 11 p+ 10 e 2 energy levels F-1 9 p+ 10 e 2 energy levels All have same amount of energy levels and electrons Answer depends on # of protons in relation to the atomic radius trend (the more protons you have, the stronger the attraction, so the smaller the atom) Answer: Mg+2, Na+1, Ne, F-1, O-2
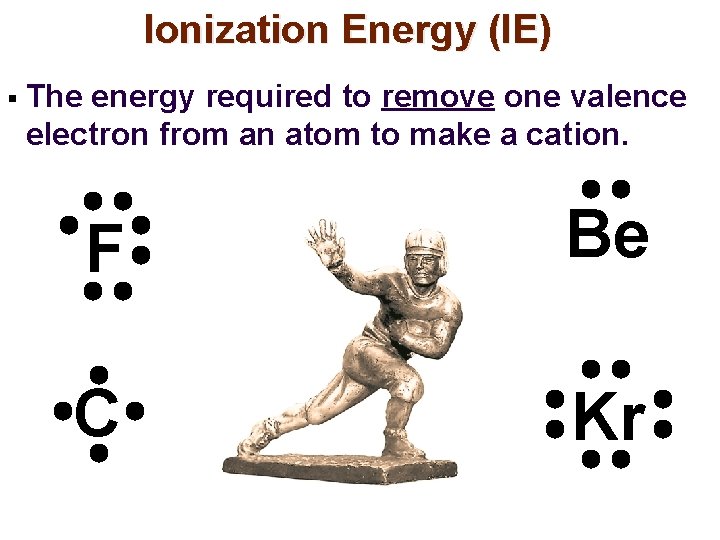
Ionization Energy (IE) § The energy required to remove one valence electron from an atom to make a cation. F Be C Kr
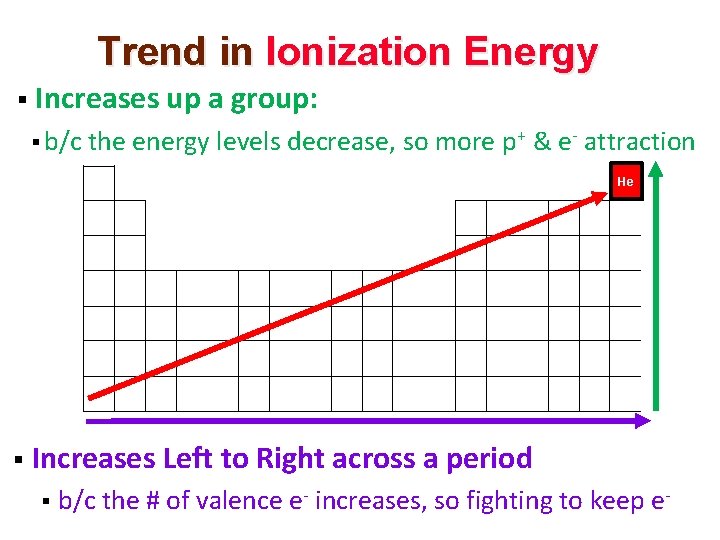
Trend in Ionization Energy § Increases up a group: § b/c the energy levels decrease, so more p+ & e- attraction He § Increases Left to Right across a period § b/c the # of valence e- increases, so fighting to keep e-
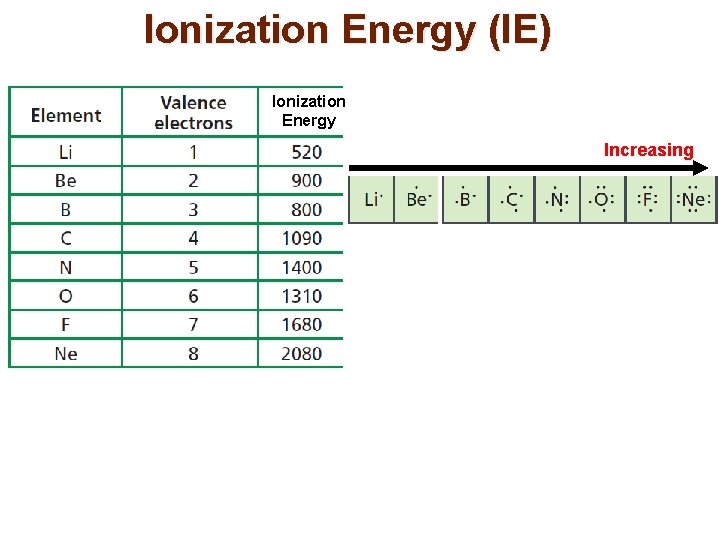
Ionization Energy (IE) Ionization Energy Increasing

Ionization Energy: Examples § Which element has a higher ionization energy? Explain. Silicon or Sulfur S requires more E to remove an ebecause has 6 val e-, close to desired 8 § Which element has a lower ionization energy? Explain. Barium or Zirconium Ba has more energy levels, so easier to take an e- away than Zr
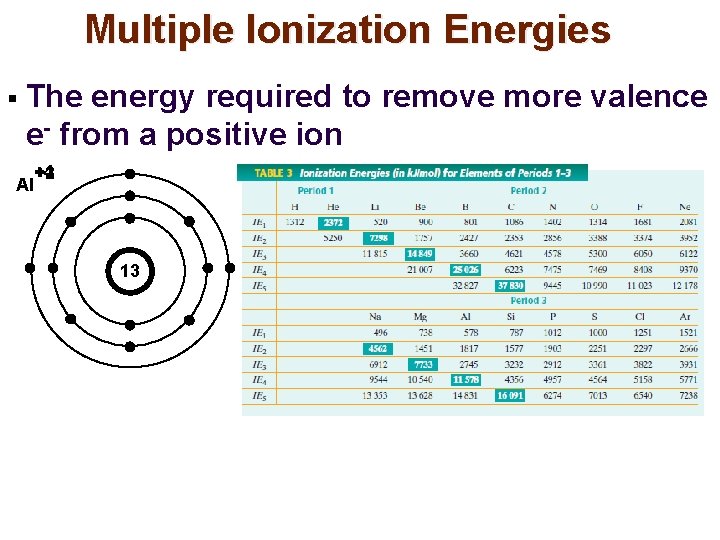
Multiple Ionization Energies § The energy required to remove more valence e- from a positive ion +4 +2 +1 +3 Al 13

Multiple Ionization Energies Example § Which ionization energy will be largest for Beryllium? Explain. 3 rd ionization energy because has 2 v. e. so once those are gone, Be has a full shell, doesn’t want to lose any more e- § Which element would have a higher 2 nd ionization energy: N or Na ? Explain. Na b/c once the 1 v. e. is removed, Na has a full outer shell, so doesn’t want to lose any more electrons, therefore more energy will be needed to remove the 2 nd e-
How the handout should look after today… AR - Distance from the nucleus to the outer shell Increases down a group b/c number of energy levels increases IE - The energy required to remove one valence electron from an atom to make a cation. Increases up a group: b/c the energy levels decrease, so more p+ & e- attraction He Fr Increases Right to Left across a period b/c of less p+ attraction from nucleus to surrounding e. Neutral Atom > Cation Anion > Neutral Atom Increases Left to Right across a period b/c the # of valence eincreases, so fighting to keep e Multiple Ionization Energies - the energy required to remove more valence e- from a positive ion
- Slides: 17