Periodic Table Trends 3 states of matter Liquid

Periodic Table Trends
3 states of matter Liquid: Hg and Br Gas: all the noble gases (group 18) and O, F, N, H, Cl Solid: rest of the elements in the periodic table

All elements are classified as either: Metals Nonmetals Semi-metals/metalloids
Metals Left and middle of periodic table except H Properties: Ductile: drawn into a wire Malleable: hammered into thin sheets Good conductors of heat/electricity Luster: shine Solid at room temperature (except for Hg)
Nonmetals Far right side of periodic table Properties Brittle: break when hammered Lack luster Poor conductors Solid or gas at room temperature (except Br)
Metalloids (Semi-metals) Along the black line that divides metals and nonmetals Have properties of both metals and nonmetals
Periodicity Elements in the same group have the same chemical and physical properties Example: Noble gases- Group 18 All gases All unreactive Same number of electrons in last energy level
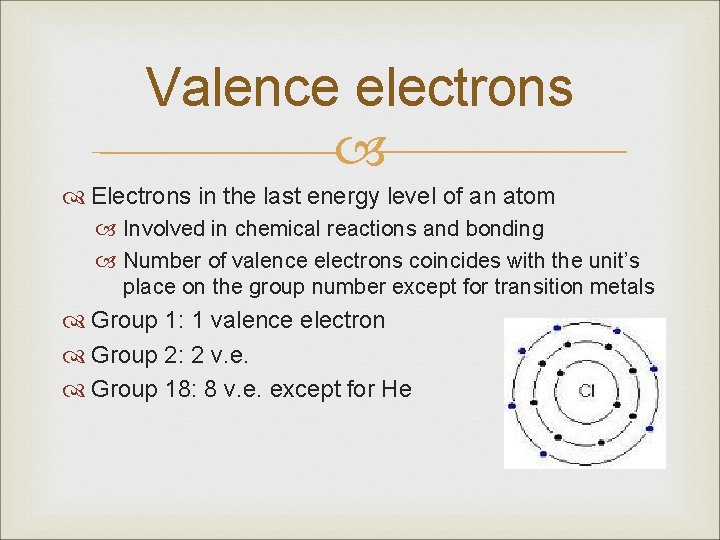
Valence electrons Electrons in the last energy level of an atom Involved in chemical reactions and bonding Number of valence electrons coincides with the unit’s place on the group number except for transition metals Group 1: 1 valence electron Group 2: 2 v. e. Group 18: 8 v. e. except for He
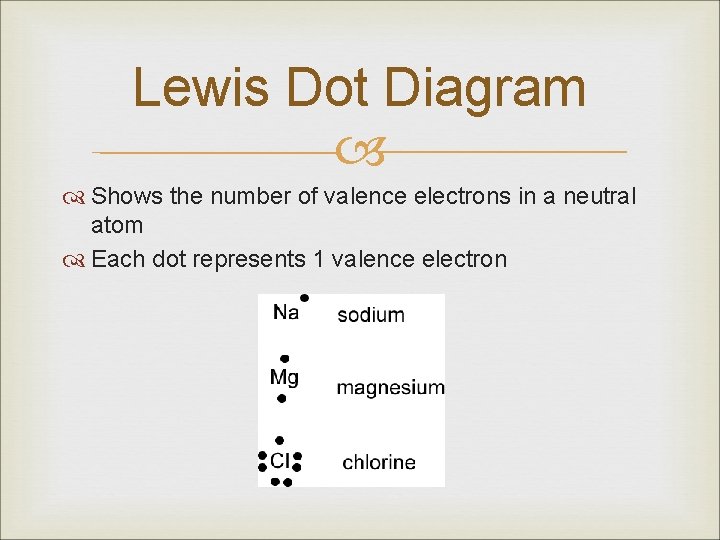
Lewis Dot Diagram Shows the number of valence electrons in a neutral atom Each dot represents 1 valence electron
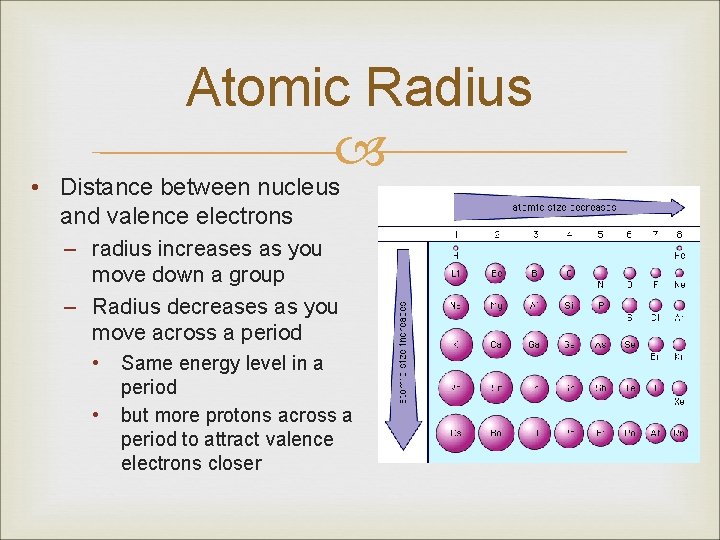
Atomic Radius • Distance between nucleus and valence electrons – radius increases as you move down a group – Radius decreases as you move across a period • • Same energy level in a period but more protons across a period to attract valence electrons closer
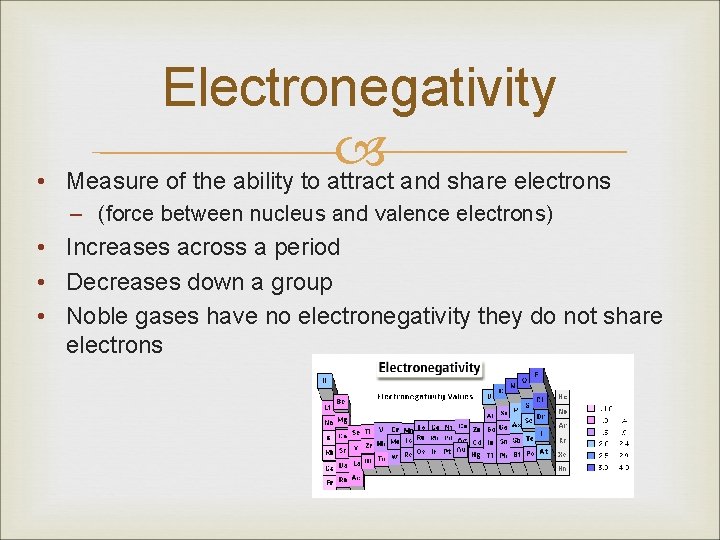
• Electronegativity Measure of the ability to attract and share electrons – (force between nucleus and valence electrons) • Increases across a period • Decreases down a group • Noble gases have no electronegativity they do not share electrons
- Slides: 11