PERIODIC TABLE TRENDS 1 Atomic Radius the electron
PERIODIC TABLE TRENDS
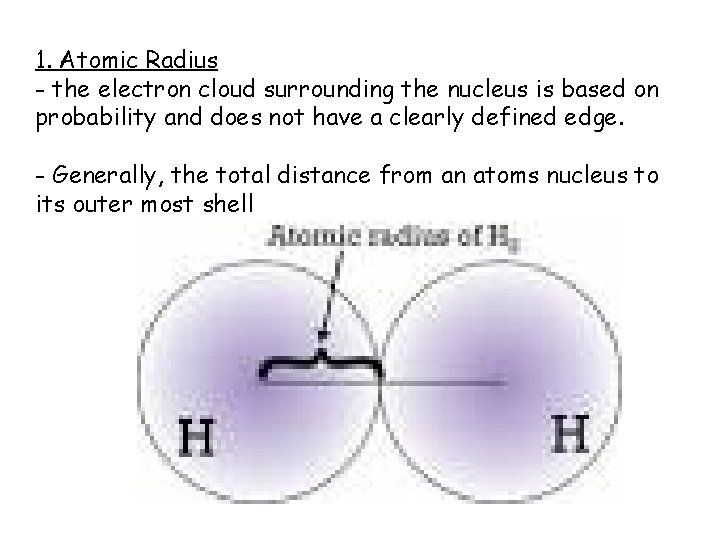
1. Atomic Radius - the electron cloud surrounding the nucleus is based on probability and does not have a clearly defined edge. - Generally, the total distance from an atoms nucleus to its outer most shell
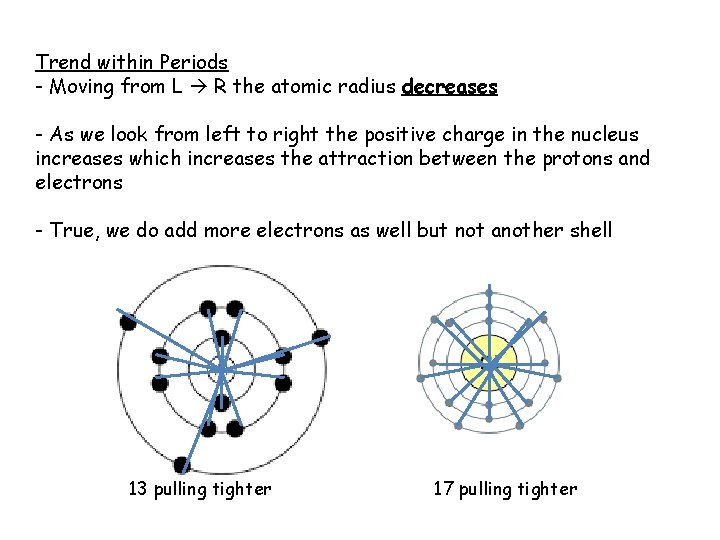
Trend within Periods - Moving from L R the atomic radius decreases - As we look from left to right the positive charge in the nucleus increases which increases the attraction between the protons and electrons - True, we do add more electrons as well but not another shell 13 pulling tighter 17 pulling tighter
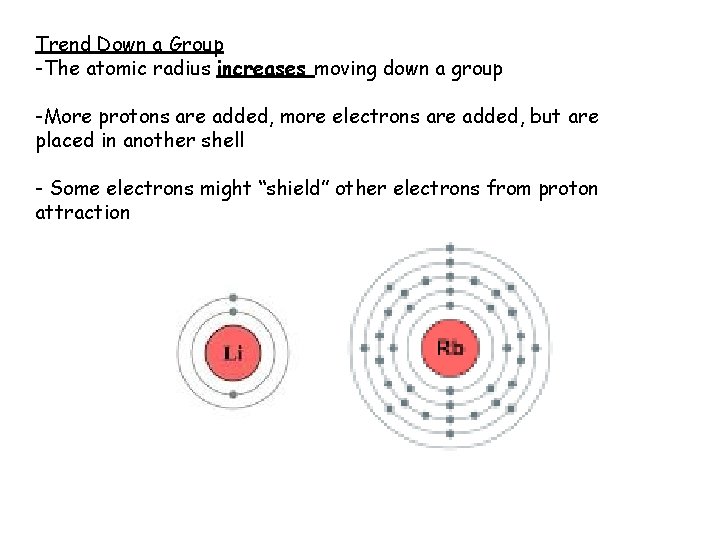
Trend Down a Group -The atomic radius increases moving down a group -More protons are added, more electrons are added, but are placed in another shell - Some electrons might “shield” other electrons from proton attraction
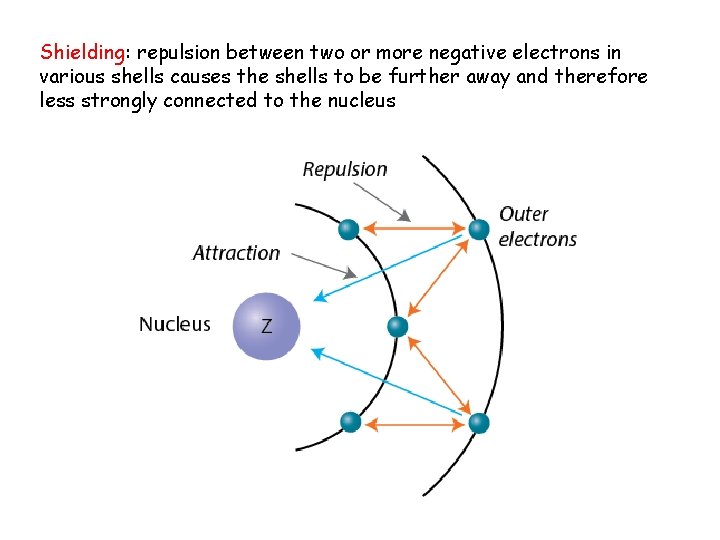
Shielding: repulsion between two or more negative electrons in various shells causes the shells to be further away and therefore less strongly connected to the nucleus
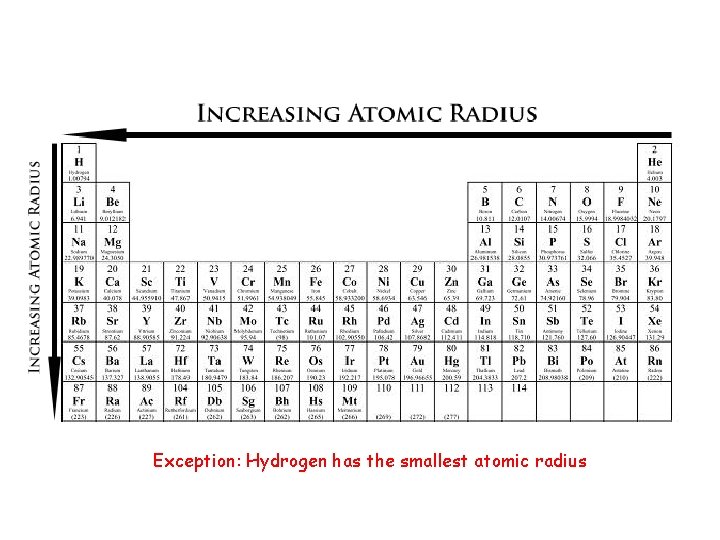
Exception: Hydrogen has the smallest atomic radius
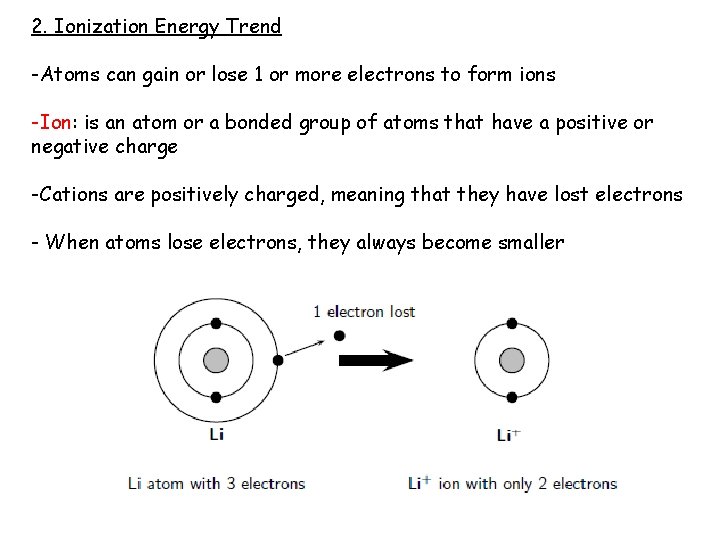
2. Ionization Energy Trend -Atoms can gain or lose 1 or more electrons to form ions -Ion: is an atom or a bonded group of atoms that have a positive or negative charge -Cations are positively charged, meaning that they have lost electrons - When atoms lose electrons, they always become smaller
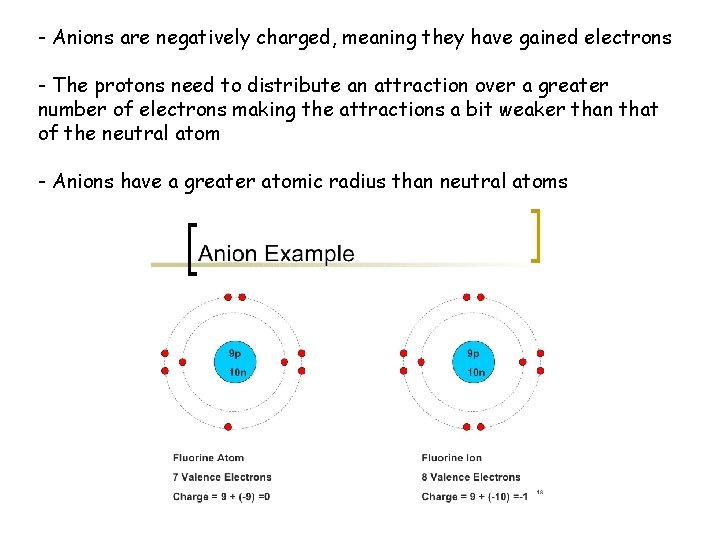
- Anions are negatively charged, meaning they have gained electrons - The protons need to distribute an attraction over a greater number of electrons making the attractions a bit weaker than that of the neutral atom - Anions have a greater atomic radius than neutral atoms
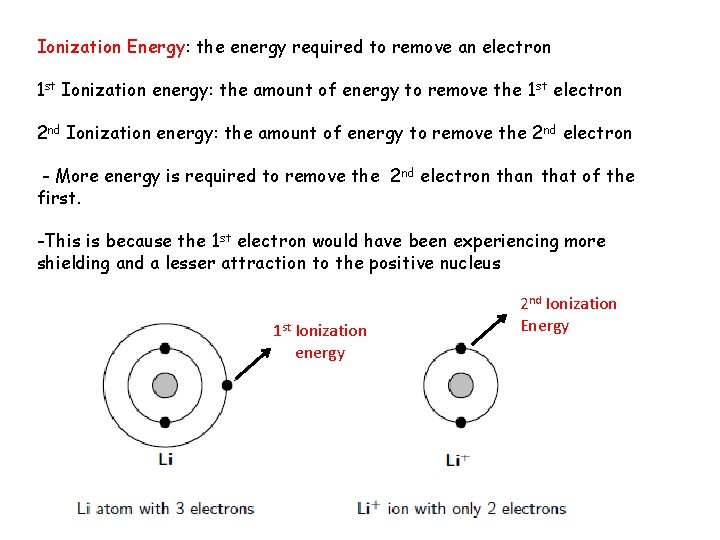
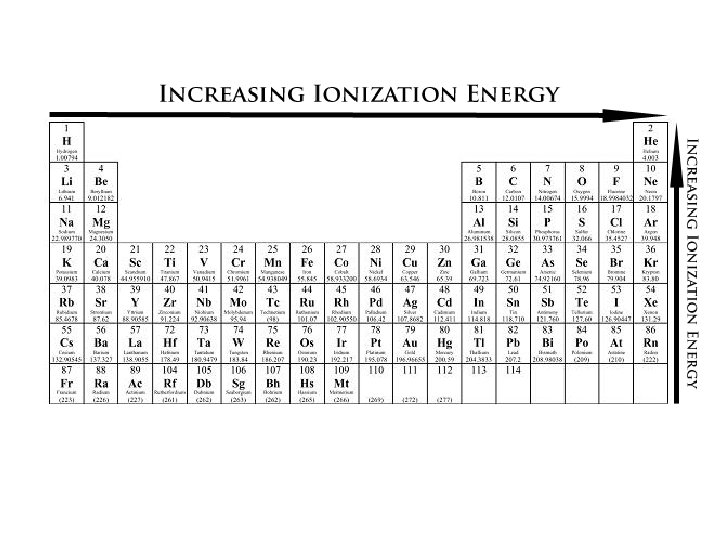
Ionization Energy: the energy required to remove an electron 1 st Ionization energy: the amount of energy to remove the 1 st electron 2 nd Ionization energy: the amount of energy to remove the 2 nd electron - More energy is required to remove the 2 nd electron that of the first. -This is because the 1 st electron would have been experiencing more shielding and a lesser attraction to the positive nucleus 1 st Ionization energy 2 nd Ionization Energy
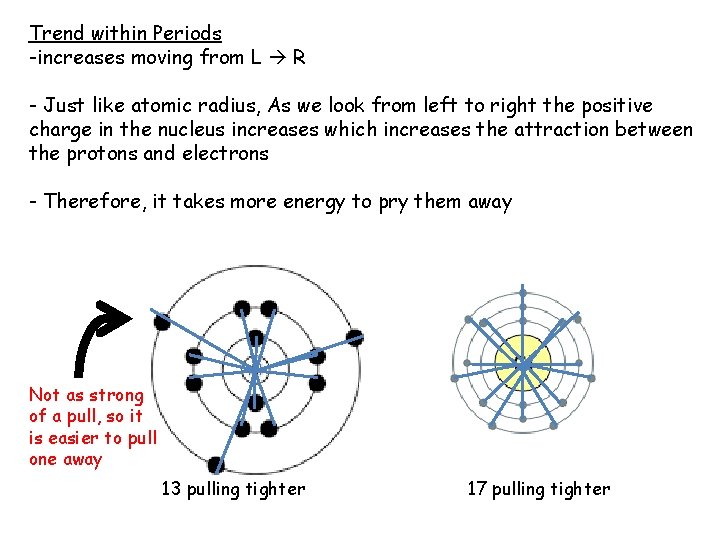
Trend within Periods -increases moving from L R - Just like atomic radius, As we look from left to right the positive charge in the nucleus increases which increases the attraction between the protons and electrons - Therefore, it takes more energy to pry them away Not as strong of a pull, so it is easier to pull one away 13 pulling tighter 17 pulling tighter
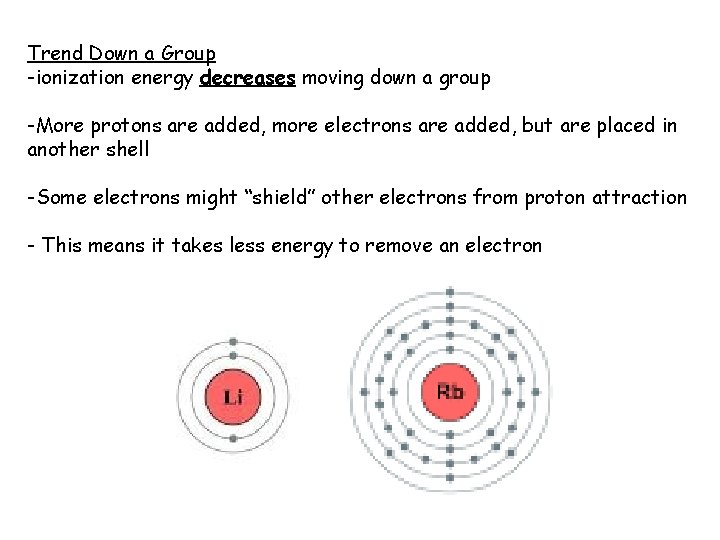
Trend Down a Group -ionization energy decreases moving down a group -More protons are added, more electrons are added, but are placed in another shell -Some electrons might “shield” other electrons from proton attraction - This means it takes less energy to remove an electron
-Mom has too many kids to watch and can’t keep a close eye on them all - as she has less kids to watch, it would be more difficult to nab them
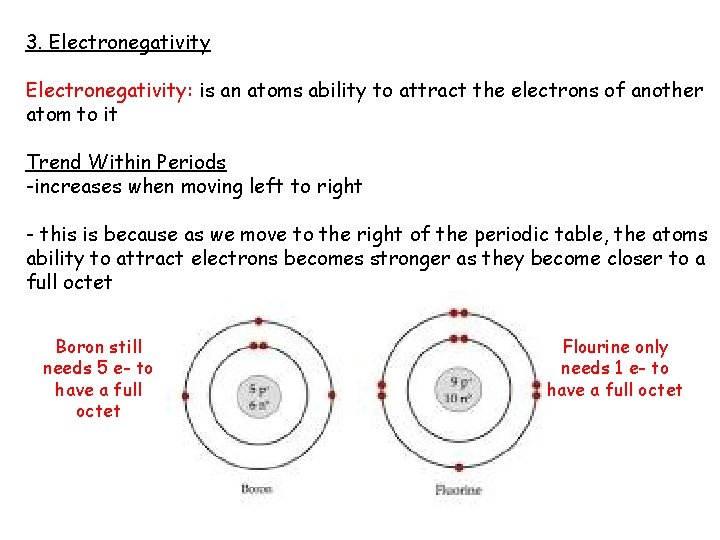
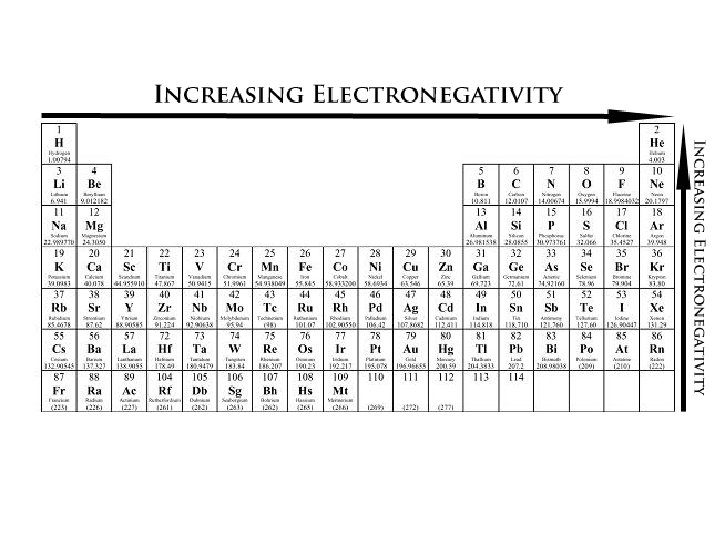
3. Electronegativity: is an atoms ability to attract the electrons of another atom to it Trend Within Periods -increases when moving left to right - this is because as we move to the right of the periodic table, the atoms ability to attract electrons becomes stronger as they become closer to a full octet Boron still needs 5 e- to have a full octet Flourine only needs 1 e- to have a full octet
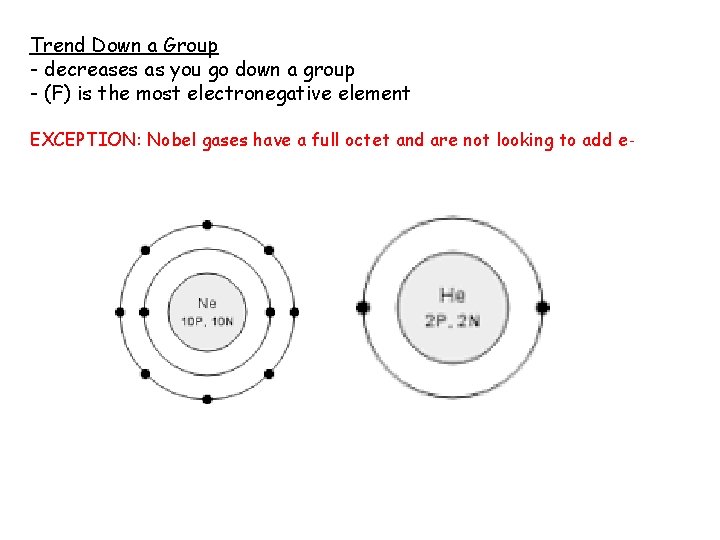
Trend Down a Group - decreases as you go down a group - (F) is the most electronegative element EXCEPTION: Nobel gases have a full octet and are not looking to add e-
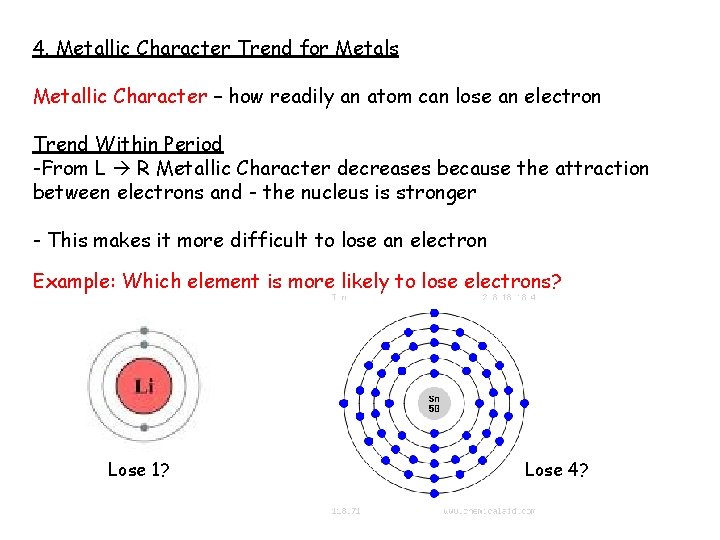
4. Metallic Character Trend for Metals Metallic Character – how readily an atom can lose an electron Trend Within Period -From L R Metallic Character decreases because the attraction between electrons and - the nucleus is stronger - This makes it more difficult to lose an electron Example: Which element is more likely to lose electrons? Lose 1? Lose 4?
Trend Down a Group -Metallic Character increases down a group -Again, like atomic radius trend, More protons are added, more electrons are added, but are placed in another shell -Some electrons might “shield” other electrons from proton attraction - This makes it easier for an electron to be lost


5. Reactivity for Metals Trend Reactivity: how likely or vigorously an atom is to react with other substances -Usually determined by how easily electrons can be removed (Same as Metal Character) Trend Within Period - From L R reactivity decreases because the attraction between electrons and the nucleus is stronger -This makes it more difficult to lose an electron, therefore less reactive

Trend Down a Group -Reactivity increases down a group -Again, like atomic radius trend, More protons are added, more electrons are added, but are placed in another shell -Some electrons might “shield” other electrons from proton attraction - This makes it easier for an electron to be lost, or become more reactive Halogen Reactivity
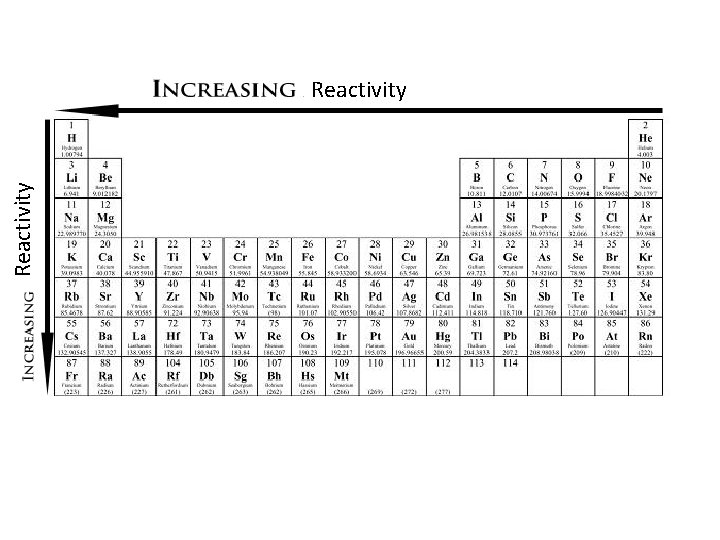
Reactivity
- Slides: 22