Periodic Table Review The periodic table is a

Periodic Table Review
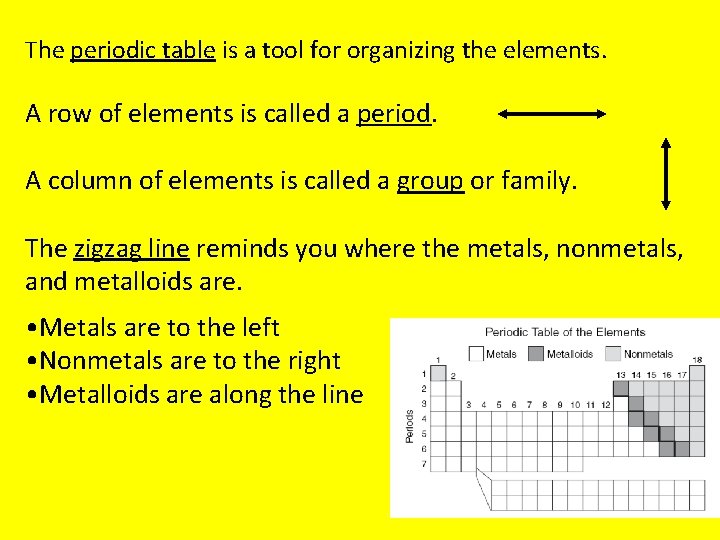
The periodic table is a tool for organizing the elements. A row of elements is called a period. A column of elements is called a group or family. The zigzag line reminds you where the metals, nonmetals, and metalloids are. • Metals are to the left • Nonmetals are to the right • Metalloids are along the line
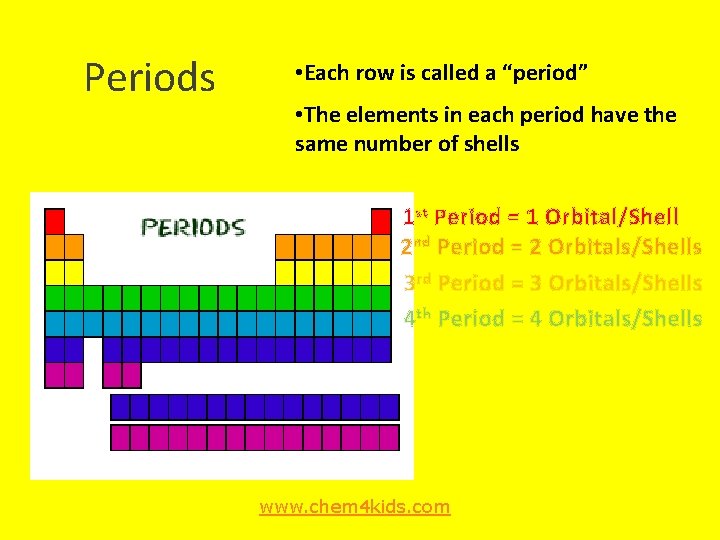
Periods • Each row is called a “period” • The elements in each period have the same number of shells 1 st Period = 1 Orbital/Shell 2 nd Period = 2 Orbitals/Shells 3 rd Period = 3 Orbitals/Shells 4 th Period = 4 Orbitals/Shells www. chem 4 kids. com
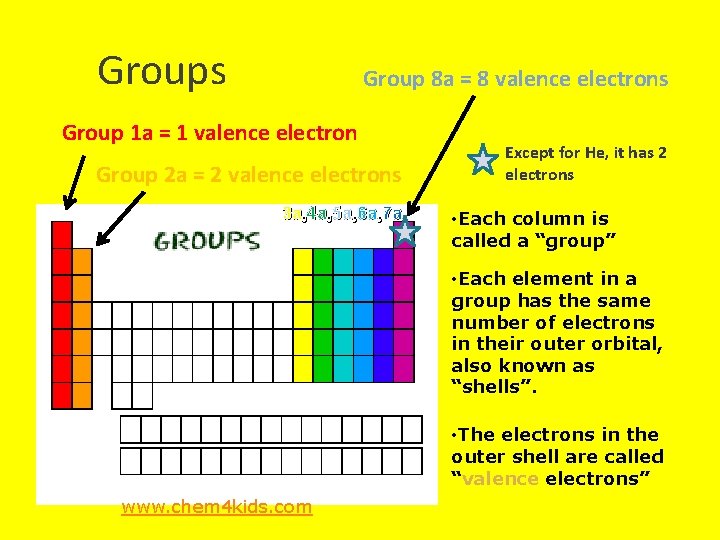
Groups Group 8 a = 8 valence electrons Group 1 a = 1 valence electron Group 2 a = 2 valence electrons 3 a, 4 a, 5 a, 6 a, 7 a Except for He, it has 2 electrons • Each column is called a “group” • Each element in a group has the same number of electrons in their outer orbital, also known as “shells”. • The electrons in the outer shell are called “valence electrons” www. chem 4 kids. com
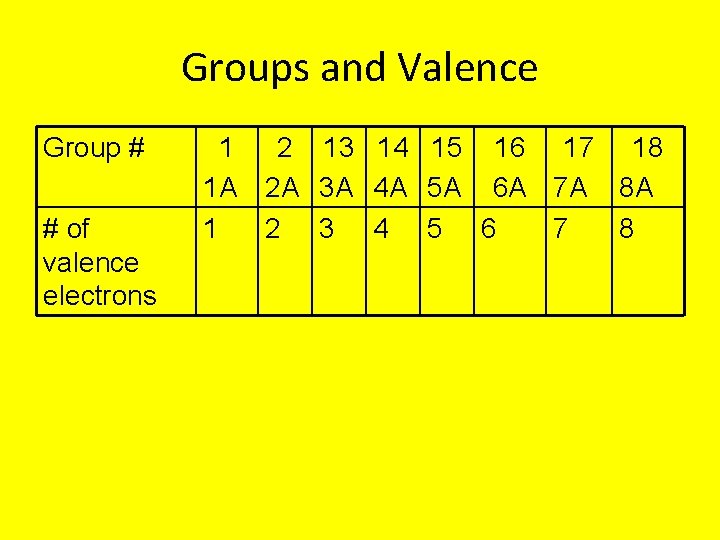
Groups and Valence Group # # of valence electrons 1 1 A 1 2 13 14 15 16 17 18 2 A 3 A 4 A 5 A 6 A 7 A 8 A 2 3 4 5 6 7 8

Discussion: Groups and Valence Electrons • The elements in a group have similar properties and react/behave in similar ways. • Valence electrons are those located in the outermost energy level of an atom – Atoms are “happy” and stable when their outermost energy level is full – all of the Noble gases in group 18 are this way. – Atoms are reactive with other atoms when their outermost energy level is not full. – Atoms in the same group have the same # of valence electrons except the transition metals which do not follow the same pattern – Elements in a family increase in reactivity as you move down.
Transition Metals • Transition Metals have slightly different rules for shells and valence electrons. • This is something you will learn about in High School Chemistry. www. chem 4 kids. com

• Alkali Metals - 1 valence electron – Most reactive group of metals • Alkaline Earth Metals - 2 valence electrons. Very reactive metals • Halides/Halogens - 7 valence electrons – Most reactive group of non-metal • Noble Gases - Complete outer shells – Most un-reactive elements
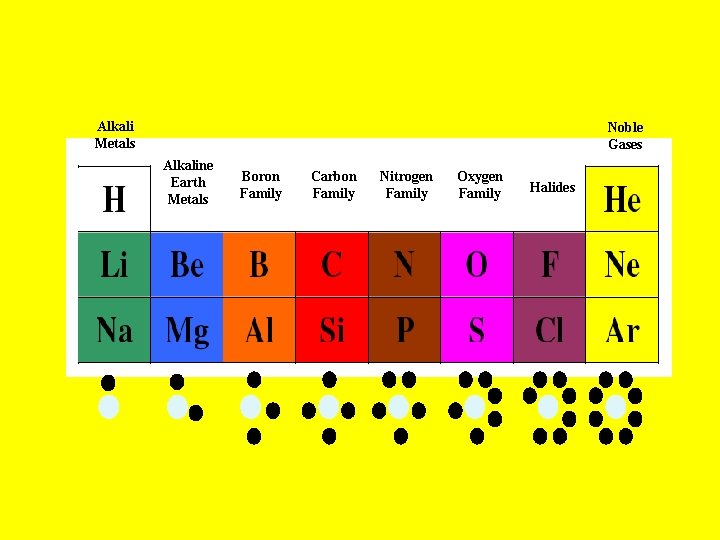
Alkali Metals Noble Gases Alkaline Earth Metals Boron Family Carbon Family Nitrogen Family Oxygen Family Halides

Time for…. . BINGO

I AM IN GROUP 2 AND PERIOD 3
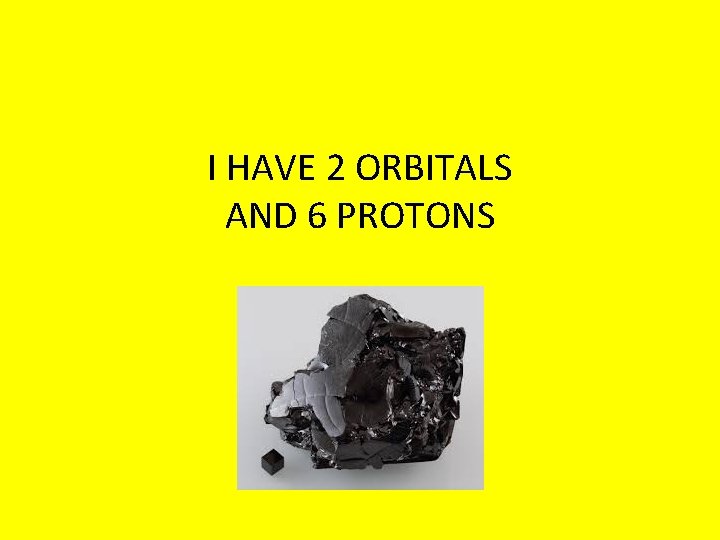
I HAVE 2 ORBITALS AND 6 PROTONS

I HAVE 9 ELECTRONS AND AM IN GROUP 17

I HAVE 7 VALENCE ELECTRONS AND AM IN PERIOD 3

I HAVE A SIMILAR REACTIVITY TO HELIUM AND 18 PROTONS

I HAVE 0 NEUTRONS, 1 PROTON, AND AM IN GROUP 1

I HAVE A SIMILAR REACTIVITY TO HYDROGEN AND 19 PROTONS

I AM A NON-METAL IN PERIOD 3 AND HAVE 6 VALENCE ELECTRONS

I AM A METAL WITH 24 ELECTRONS

I HAVE TWO VALENCE ELECTRONS AND 4 ORBITALS

I HAVE A ROUNDED ATOMIC MASS OF 7 AND I AM IN GROUP 1

I AM A METAL IN PERIOD 4 WITH A ROUNDED ATOMIC MASS OF 48

I HAVE 5 VALENCE ELECTRONS AND 15 TOTAL ELECTRONS

I AM IN PERIOD 2 AND HAVE 5 NEUTRONS

I AM A NOBLE GAS, I AM VERY UNREACTIVE, AND I HAVE 10 ELECTRONS

I AM IN GROUP 13, PERIOD 2

I AM IN PERIOD 3 AND HAVE A ROUNDED ATOMIC MASS OF 27

I LIKE TO COMBINE WITH HYDROGEN. I HAVE 6 VALENCE ELECTRONS AND 8 ELECTRONS

I AM IN PERIOD 3 AND AM IN THE MOST REACTIVE GROUP OF METALS

I AM VERY UN-REACTIVE, I HAVE 2 VALENCE ELECTRONS BUT I AM IN GROUP 18

I HAVE 4 ORBITALS AND 23 PROTONS

I HAVE 4 VALENCE ELECTRONS AND 14 ELECTRONS

I HAVE 4 ORBITALS AND A ROUNDED ATOMIC MASS OF 45

I HAVE 5 VALENCE ELECTRONS AND AM IN PERIOD 2
- Slides: 34