Periodic Table Periodic Table Origins 1860 60 elements
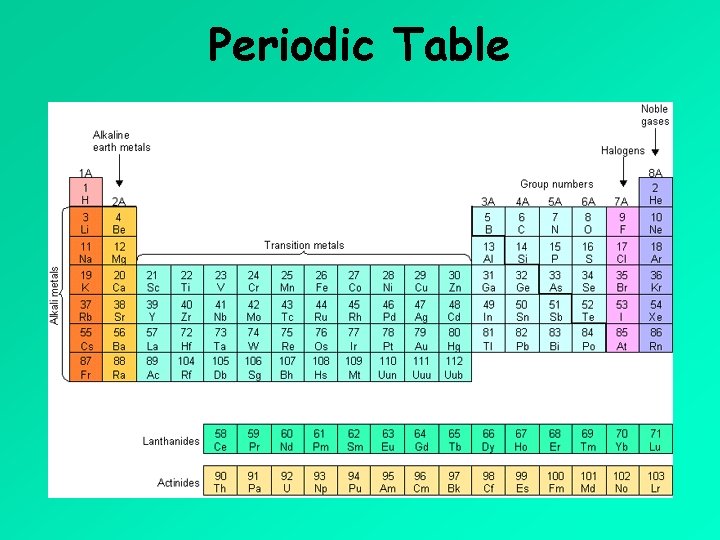
Periodic Table
Periodic Table Origins • 1860: ~60 elements had been identified • need for organization of elements was recognized

Dmitri Mendeleev • • • created version in 1869, revised version in 1872 first attempt to organize elements into a logical format arranged elements by increasing atomic mass spaces left for missing elements attempted to keep elements together by similar chemical properties as well • these two ideas could not both be followed correctly at the same time
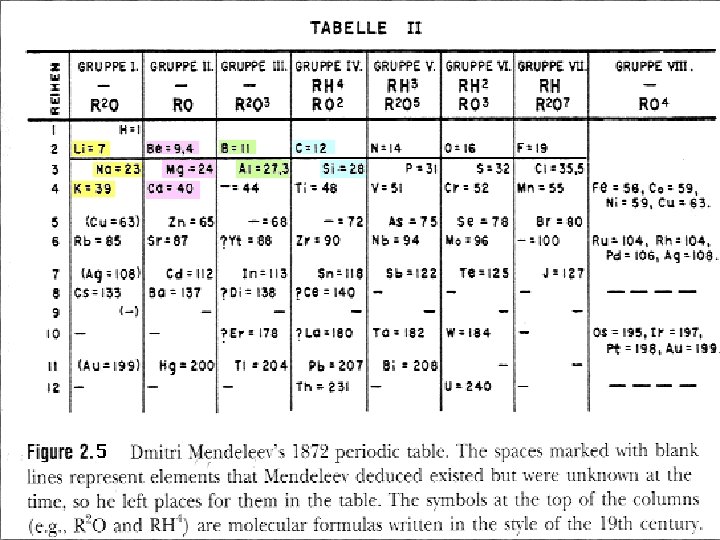


Henry Moseley • created current version in 1911 • identified atomic number by determining relationship between x-ray frequency and number of protons • arranged elements by increasing atomic number (number of protons in the nucleus) • elements with similar chemical properties stayed together in vertical groups using this format
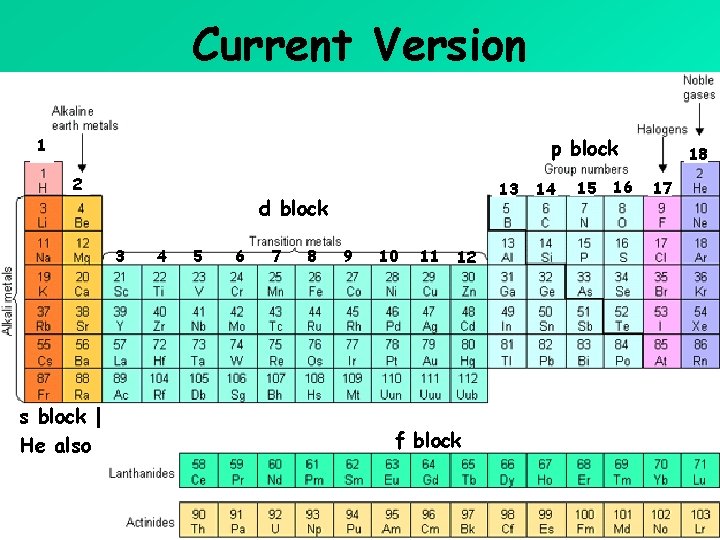
Current Version p block 1 2 d block 3 s block | He also 13 4 5 6 7 8 9 10 11 12 f block 14 15 16 18 17

Periodic Law • Mendeleev’s and Moseley’s work, along with many other scientists, led to the formation of the Periodic Law - many of the physical and chemical properties of the elements tend to occur in a systematic manner with increasing atomic number - elements that have similar chemical properties fall within the same group or family of elements on the periodic table.

Classifying Elements
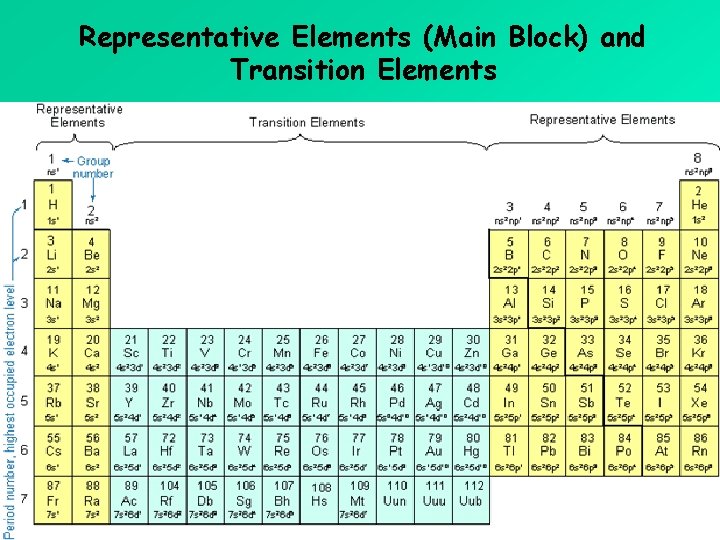
Representative Elements (Main Block) and Transition Elements
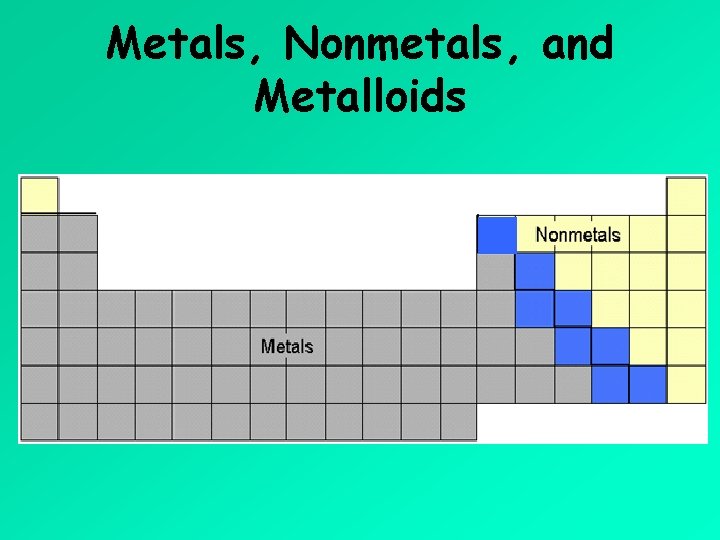
Metals, Nonmetals, and Metalloids

Metals • majority of elements (over 2/3 on left hand side of periodic table) are classified as metals • tend to lose electrons when forming compounds (form cations) • have similar physical properties - can efficiently conduct electricity and heat - can be easily shaped (malleability) - can be pulled into thin wires (ductility) - have a shiny appearance (lustrous) - majority are found in solid state of matter at room temperature (exception: mercury (Hg) is a liquid and gallium will melt in your hand)

Nonmetals • small number of elements on right hand side of periodic table • tend to gain or share electrons when forming compounds (When they gain electrons, they form anions. ) • physical properties vary but in general are opposite to those of metals - do not efficiently conduct electricity and heat (insulators) - cannot be easily shaped or pulled into thin wires (brittle when solid) - do not have a shiny appearance (dull surface) - most are found in gaseous state at room temperature - one is found as a liquid (bromine) - several are found as solids (iodine, phosphorus, carbon, and sulfur)

Metalloids • found adjacent to “stairway” line shown on periodic table • another name for these elements is semimetals • these elements have a mixture of metal and nonmetal properties – for example, many are brittle (like nonmetals) but are shiny and conduct electricity as semiconductors. • aluminum (Al) is not classified as a metalloid (it is a metal) • some sources do not classify boron (B) or astatine (At) as metalloids • silicon and germanium are used as semiconductors in computers and other portable electronic devices
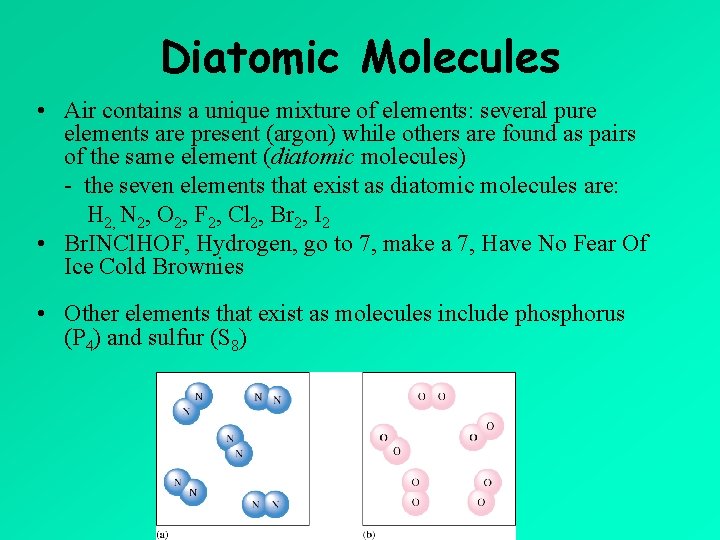
Diatomic Molecules • Air contains a unique mixture of elements: several pure elements are present (argon) while others are found as pairs of the same element (diatomic molecules) - the seven elements that exist as diatomic molecules are: H 2, N 2, O 2, F 2, Cl 2, Br 2, I 2 • Br. INCl. HOF, Hydrogen, go to 7, make a 7, Have No Fear Of Ice Cold Brownies • Other elements that exist as molecules include phosphorus (P 4) and sulfur (S 8)
Monatomic Elements • These elements include all other elements such as boron, carbon, selenium, and silicon, for example. • Others include the noble gases, which do not combine readily with other elements - these elements are classified as monatomic: they can be isolated as single atoms from nature
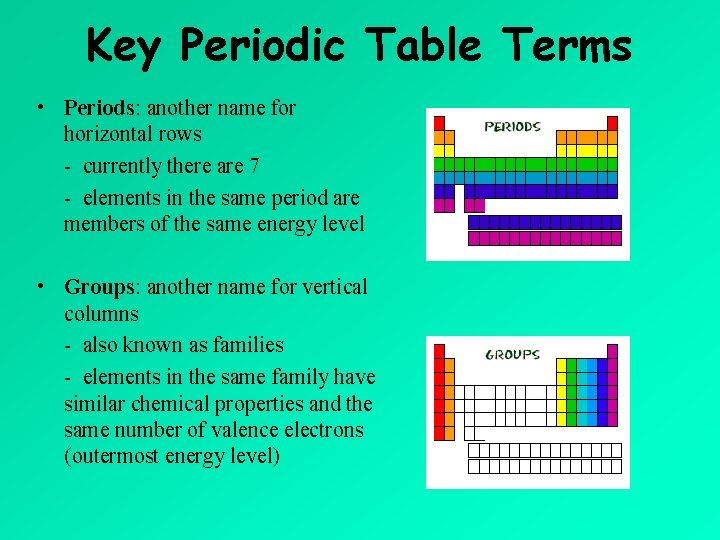
Key Periodic Table Terms • Periods: another name for horizontal rows - currently there are 7 - elements in the same period are members of the same energy level • Groups: another name for vertical columns - also known as families - elements in the same family have similar chemical properties and the same number of valence electrons (outermost energy level)

Alkali Metals • members of group 1(except H) • most reactive metals, must be stored under kerosene or oil • soft enough that they can be cut with a knife • react violently with water and acids - with water they produce H 2 gas, a metal (M+1) hydroxide, and heat • lose their only valence electron when reacting forming a cation with a +1 charge (M+1) Click on the table above for a video. Click on picture above for PTo. V Demos

Alkaline Earth Metals • members of group 2 • very reactive metals but not as reactive as the alkali metals • atoms lose both valence electrons to form ions with a +2 charge Click on the table above for a video.
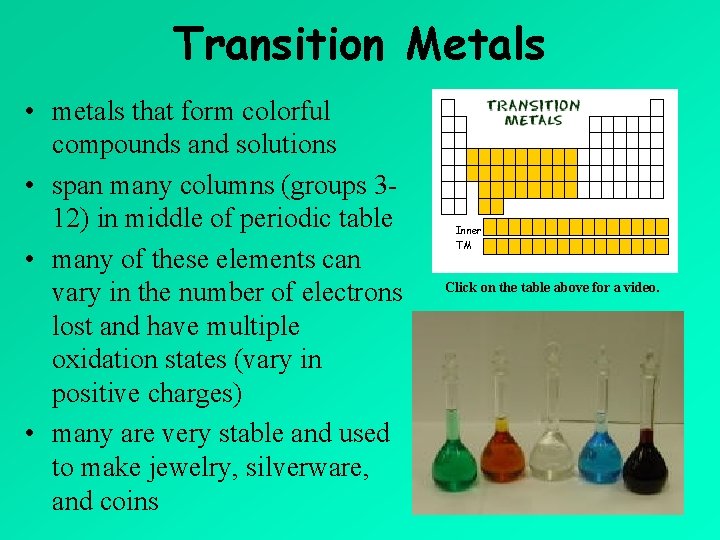
Transition Metals • metals that form colorful compounds and solutions • span many columns (groups 312) in middle of periodic table • many of these elements can vary in the number of electrons lost and have multiple oxidation states (vary in positive charges) • many are very stable and used to make jewelry, silverware, and coins Inner TM Click on the table above for a video.
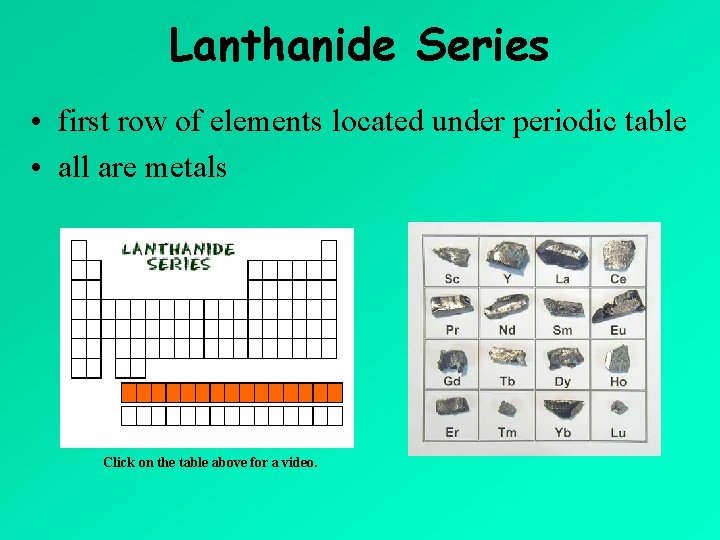
Lanthanide Series • first row of elements located under periodic table • all are metals Click on the table above for a video.
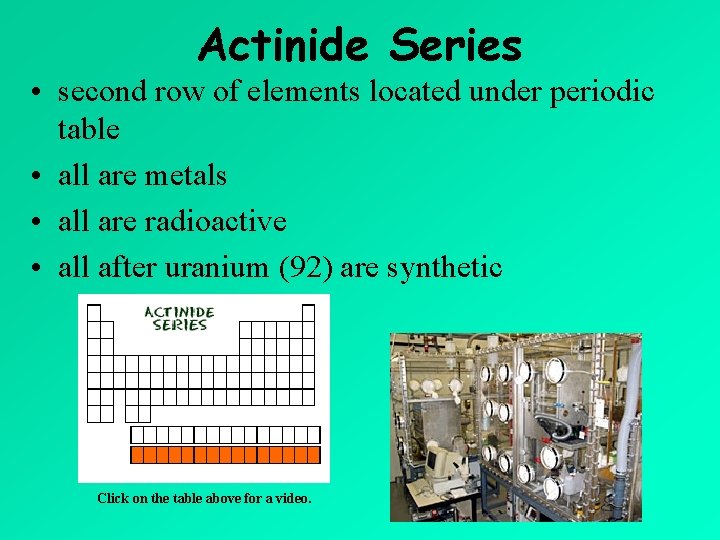
Actinide Series • second row of elements located under periodic table • all are metals • all are radioactive • all after uranium (92) are synthetic Click on the table above for a video.

Boron Family • members of group 13 • boron and aluminum are often used in commercial products • 1 metalloid, 4 metals Click on the photo above for a video.

Carbon Family • members of group 14 • 1 nonmetal, 2 metalloids, 2 metals • carbon exists as three different allotropes (different forms of the same element): diamond, graphite, and buckminsterfullerene • the branch of chemistry that studies carbon compounds is called: Organic Chemistry • silicon is essential for computers and electronics Click on the photo above for a video.

Nitrogen Family Click on the photo above for a video. • members of group 15 • 2 nonmetals, 2 metalloids and 1 metal • nitrogen and phosphorus atoms gain 3 valence electrons to form anions with a -3 charge • nitrogen makes up ~80% of Earth’s air • nitrogen is unreactive • nitrogen compounds are often explosive • phosphorus also has many applications Click on the photo above for a video.

Oxygen Family • • members of group 16 3 nonmetals, 2 metalloids polonium is radioactive oxygen, sulfur and selenium atoms gain 2 electrons to form anions with a -2 charge Click on the photo above for a video.

Halogens Click on the table above for a video. Click on the photo above for a video. • members of group 17 • most reactive nonmetals • fluorine will react with every element except He and Ne • all are diatomic in pure state • atoms gain 1 valence electron to form anions with a -1 charge • astatine is radioactive and rare Click on the photo above for a video.

Noble Gases Click on the table above for a video. • members of group 18 • least reactive nonmetals (have little to no chemical reactivity) • colorless, odorless, and tasteless monatomic gases • radon is radioactive
- Slides: 28