Periodic Table Patterns in Element Properties Some elements
Periodic Table

Patterns in Element Properties �Some elements exhibit similar chemical and physical properties. For example, lithium (Li), sodium (Na), and Potassium (K) can all combine in a 1: 1 ratio easily with Chlorine.

Elements With Similar Properties �The elements chlorine, bromine, and iodine look very different from each other. But each forms a similar-looking while solid when it reacts with sodium.
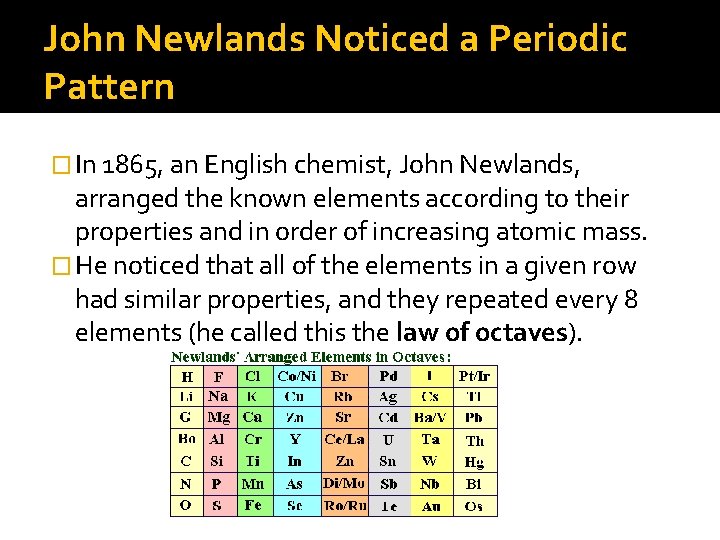
John Newlands Noticed a Periodic Pattern � In 1865, an English chemist, John Newlands, arranged the known elements according to their properties and in order of increasing atomic mass. � He noticed that all of the elements in a given row had similar properties, and they repeated every 8 elements (he called this the law of octaves).
Newlands’ Table �In Newland’s time, determining atomic weights was based on comparing other elements to the lightest element (hydrogen). Some of the elements were given inaccurate values. . �Newland was ridiculed by other chemists who felt the table he created was not reliable. He could not get his papers published and returned as chief chemist in a sugar factory and later opened a chemical business with his brother.

Dmitri Mendeleev Invented the First Periodic Table �In 1869, a Russian chemist, Dmitri Mendeleev produced the first orderly arrangement (periodic table) of all 63 elements known at the time.
Mendeleev—the Father of the Periodic Table � Mendeleev (1834 -1907) rose from very poor beginnings to a position of a renowned Russian chemist in the 19 th century. He wrote down information on each element on cards. He ranked the elements from lightest to heaviest. �Mendeleev also put the elements into a table according to their properties. He started a new row each time he noticed that the chemical properties of the elements repeated.

Mendeleev Correctly Predicted the Gaps �Mendeleev’s Table contains gaps that elements with particular properties should fill. �He correctly predicted the properties of the missing elements. He even gave them provisional names. These elements were eventually discovered.

Henry Moseley �A young English chemist, Henry Moseley, discovered that the elements should be organized according to their atomic numbers, not their atomic weights as was done before. �When Moseley studied the lines in the X-ray spectra of 38 different elements, he found that the wavelength of the lines decreased in a regular manner as atomic number increased.

Henry Moseley �Henry Moseley lost his life in 1915 during World War I at the Gallipoli battle in Turkey at the age of 27 and is buried there. His death has been called one of the greatest tragedies of WWI because he was such a brilliant chemist.

The Periodic Law � The Periodic Law states that when the elements are arranged according to their atomic numbers, elements with similar properties appear at regular intervals.
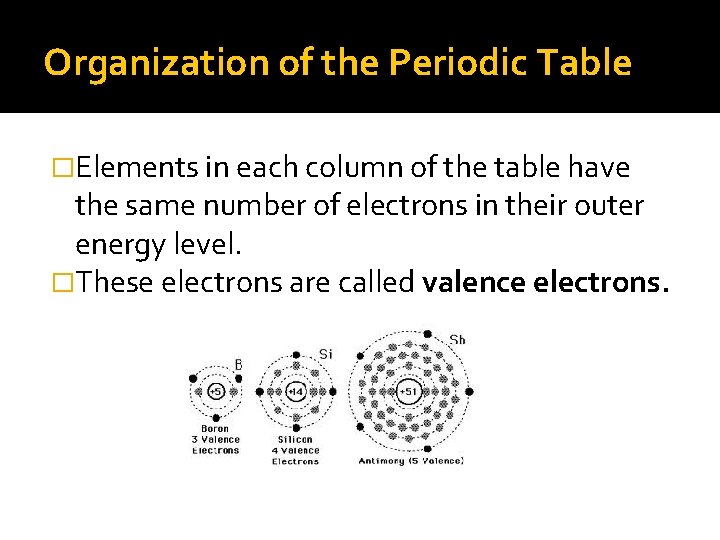
Organization of the Periodic Table �Elements in each column of the table have the same number of electrons in their outer energy level. �These electrons are called valence electrons.
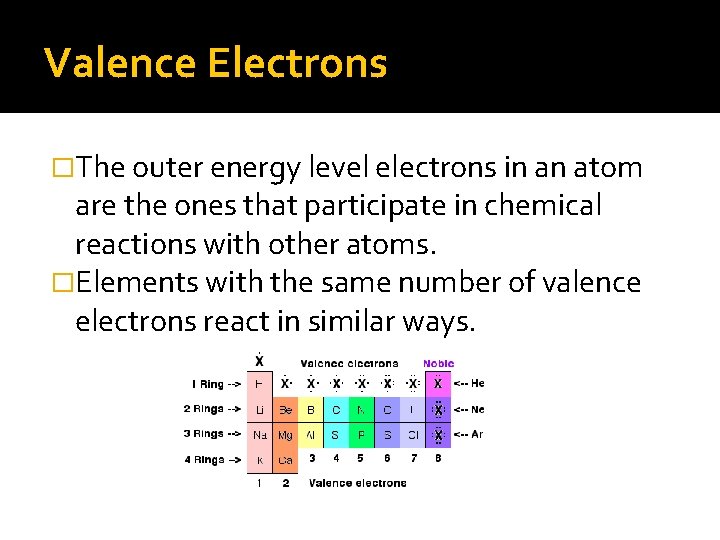
Valence Electrons �The outer energy level electrons in an atom are the ones that participate in chemical reactions with other atoms. �Elements with the same number of valence electrons react in similar ways.
Groups �A vertical column on the periodic table is called a group. (These are also called families. ) These exhibit similar properties.
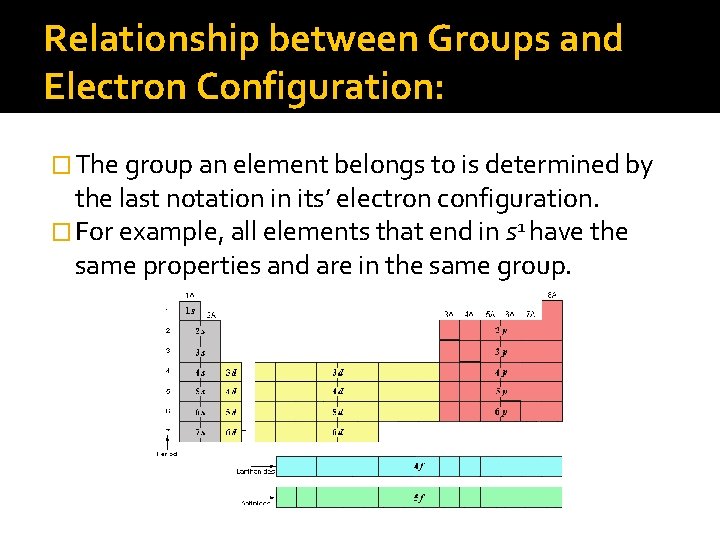
Relationship between Groups and Electron Configuration: � The group an element belongs to is determined by the last notation in its’ electron configuration. � For example, all elements that end in s 1 have the same properties and are in the same group.
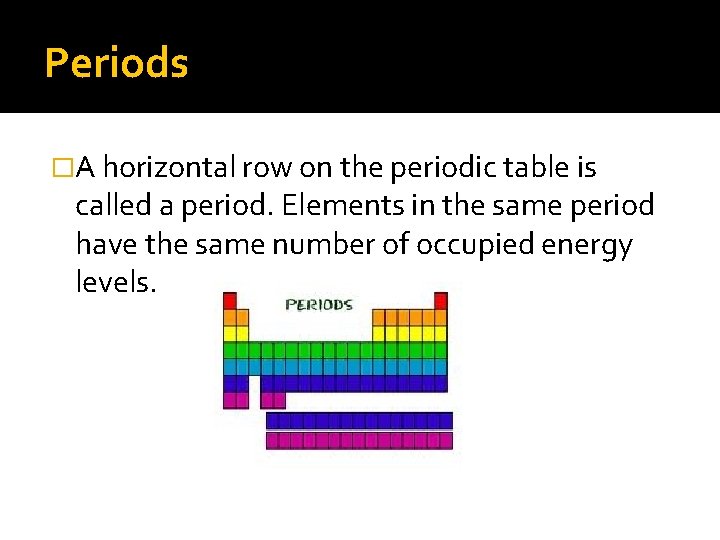
Periods �A horizontal row on the periodic table is called a period. Elements in the same period have the same number of occupied energy levels.
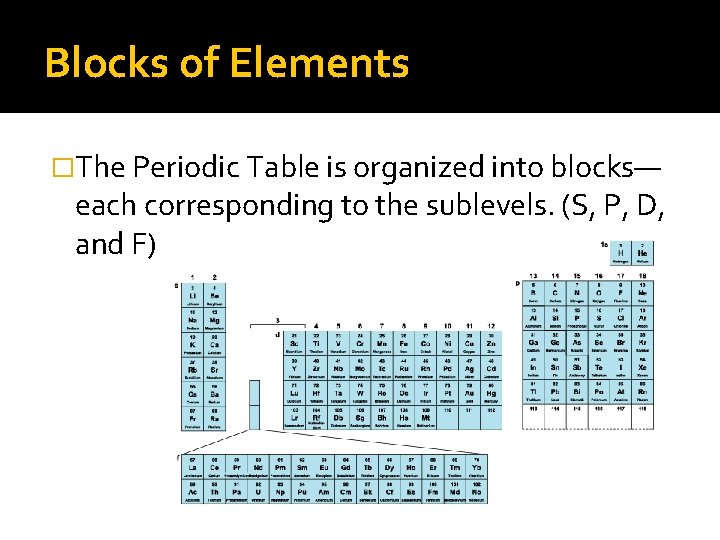
Blocks of Elements �The Periodic Table is organized into blocks— each corresponding to the sublevels. (S, P, D, and F)
- Slides: 17