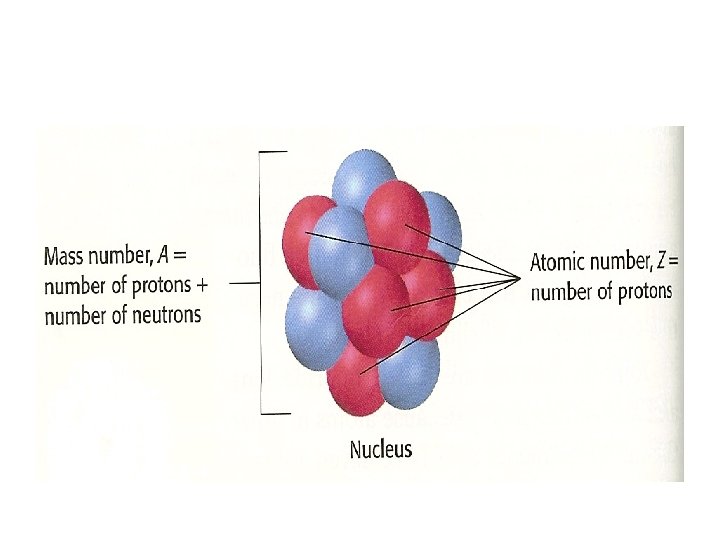
PERIODIC TABLE OF ELEMENTS Review nucleus proton neutron
PERIODIC TABLE OF ELEMENTS

• Review: - nucleus -proton - neutron - electron - Bohr’s atomic model - e- on energy level - orbitals (define the 4 type of orbitals)

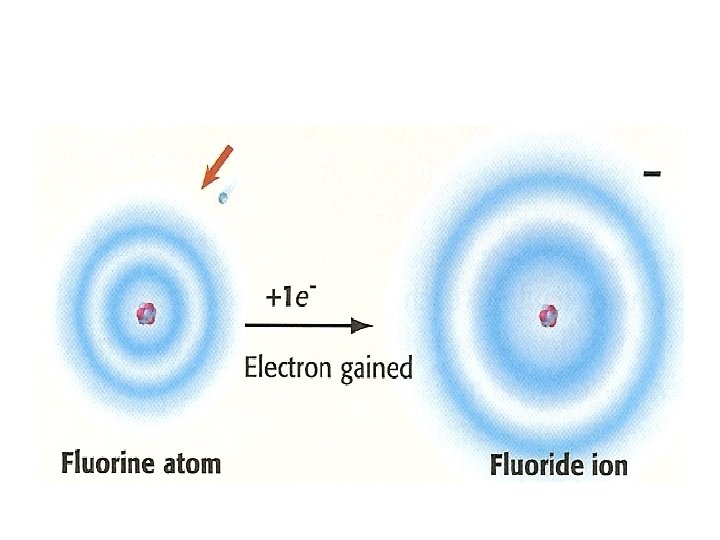
• VALENCE ELECTRON- an e- that is found in the outermost shell of an atom and that determines the atom’s chemical proprieties • Ex: in a neon (Ne) atom has 10 e-, 2 e- fills the lowest energy level, its valence e- are the 8 e- in the atom’s second (and outermost) energy level

• The Periodic table groups similar elements together • This organization predict the proprieties of an element based on where it is in the periodic table • The order is based on the number of protons an atom of that element has in its nucleus.
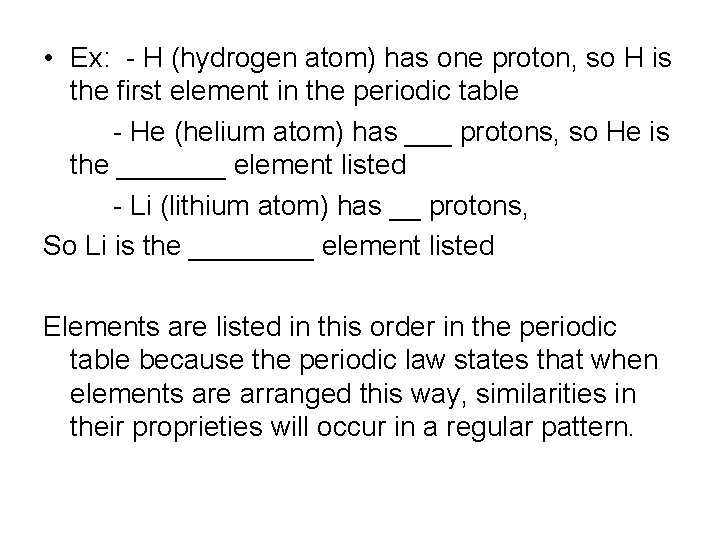
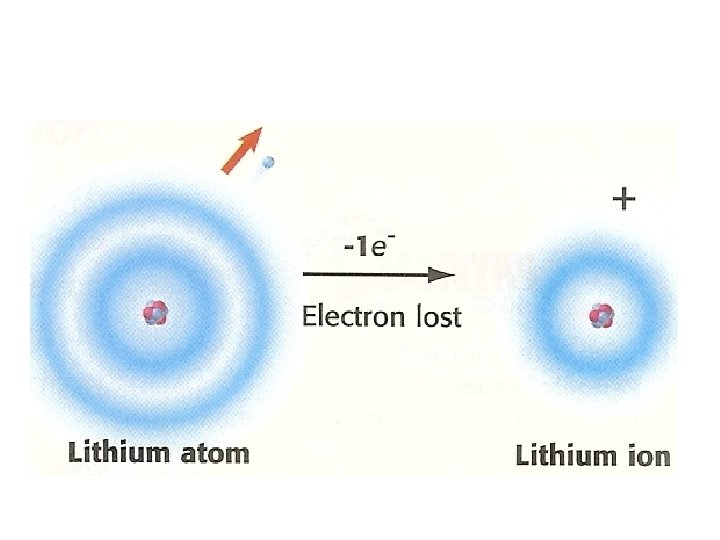
• Ex: - H (hydrogen atom) has one proton, so H is the first element in the periodic table - He (helium atom) has ___ protons, so He is the _______ element listed - Li (lithium atom) has __ protons, So Li is the ____ element listed Elements are listed in this order in the periodic table because the periodic law states that when elements are arranged this way, similarities in their proprieties will occur in a regular pattern.
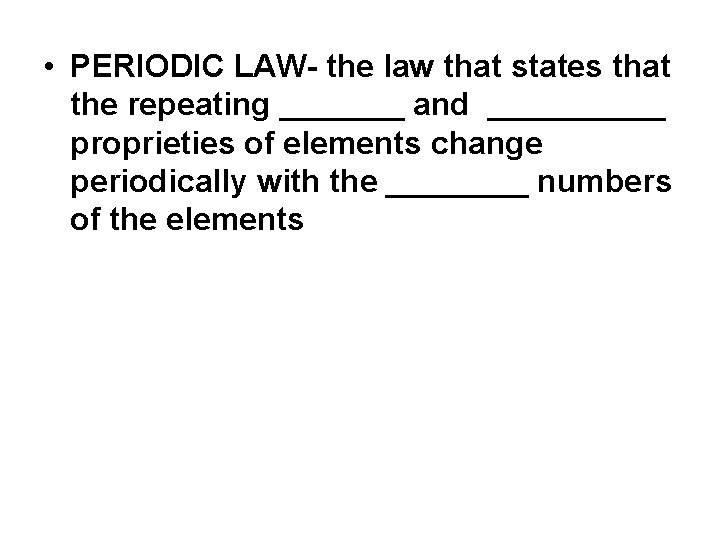
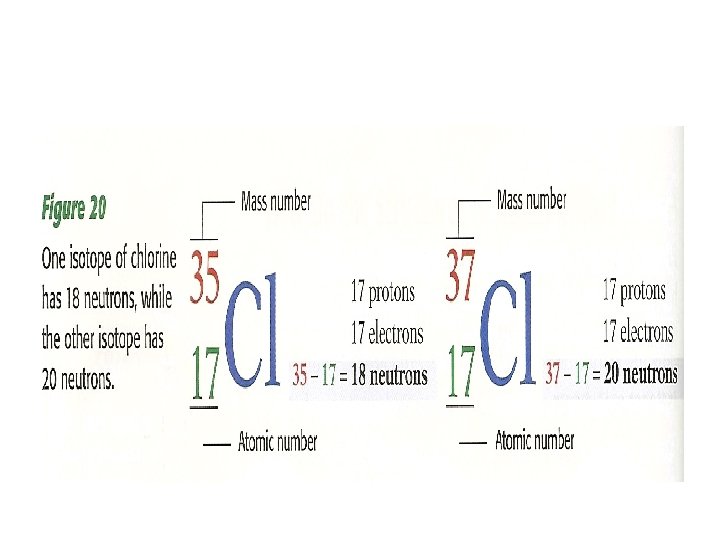
• PERIODIC LAW- the law that states that the repeating _______ and _____ proprieties of elements change periodically with the ____ numbers of the elements


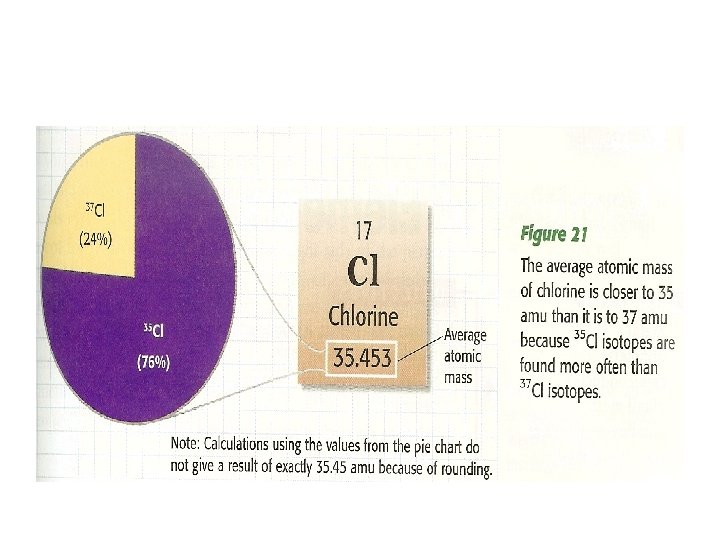
• Find the symbol for the following elements: potassium; zirconium; barium; platinum; arsenic; curium • Find the ATOMIC NUMBER of the following elements: neon; cesium; polonium; krypton • Find the name of the following elements: Sr; Au; S; Fe; Na • Find the average atomic mass for the following elements: Zn (zinc); Mn(manganese); Sr(strontium)
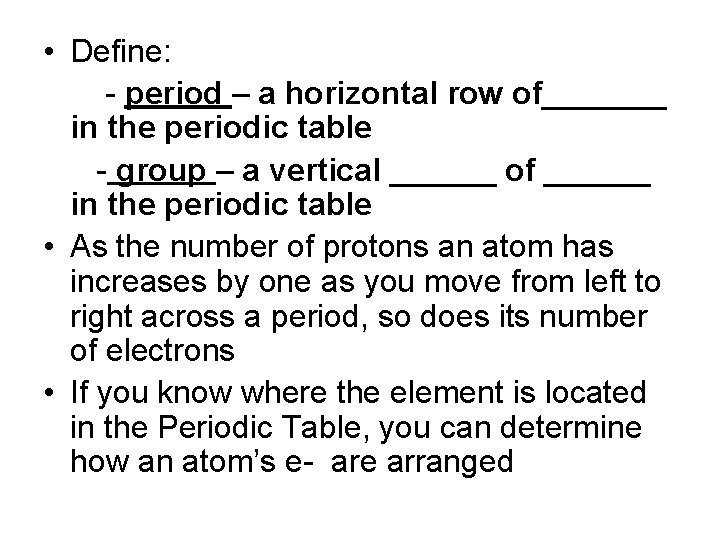
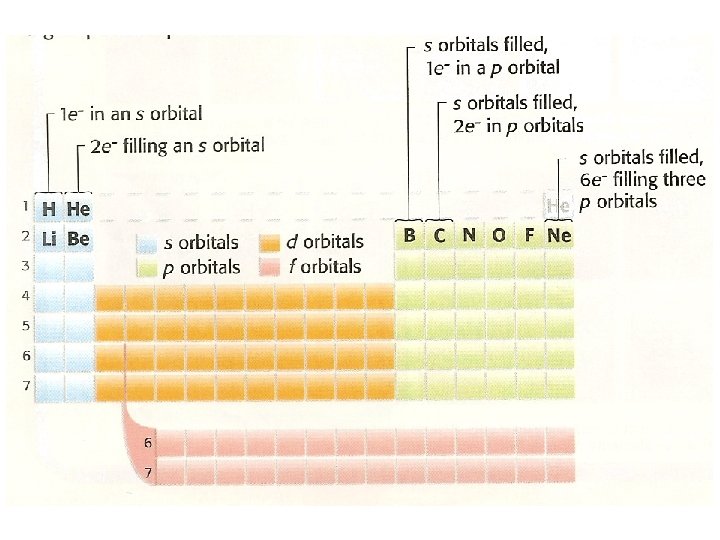
• Define: - period – a horizontal row of_______ in the periodic table - group – a vertical ______ of ______ in the periodic table • As the number of protons an atom has increases by one as you move from left to right across a period, so does its number of electrons • If you know where the element is located in the Periodic Table, you can determine how an atom’s e- are arranged
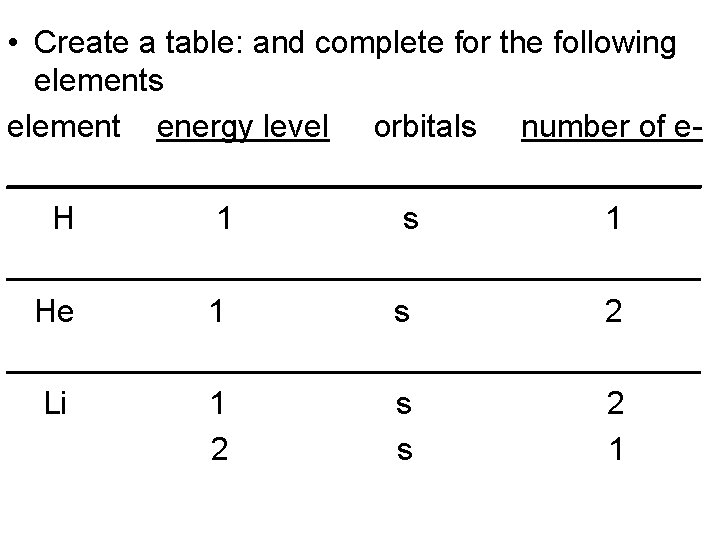
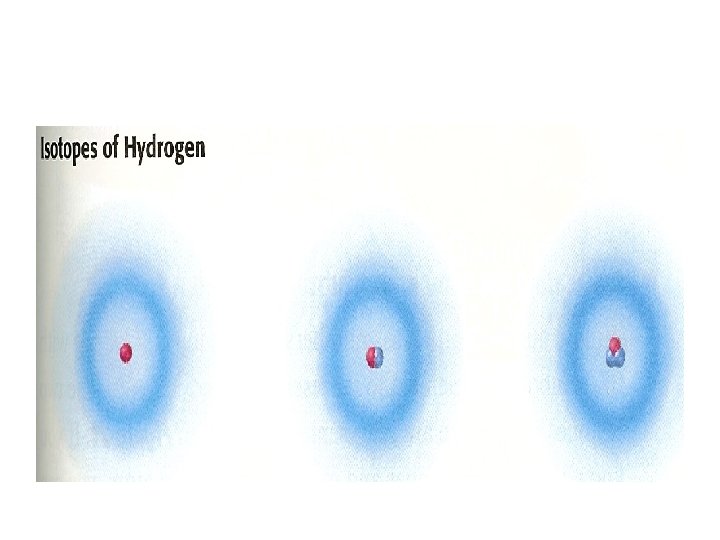
• Create a table: and complete for the following elements element energy level orbitals number of e____________________ H 1 s 1 ____________________ He 1 s 2 ____________________ Li 1 s 2 2 s 1
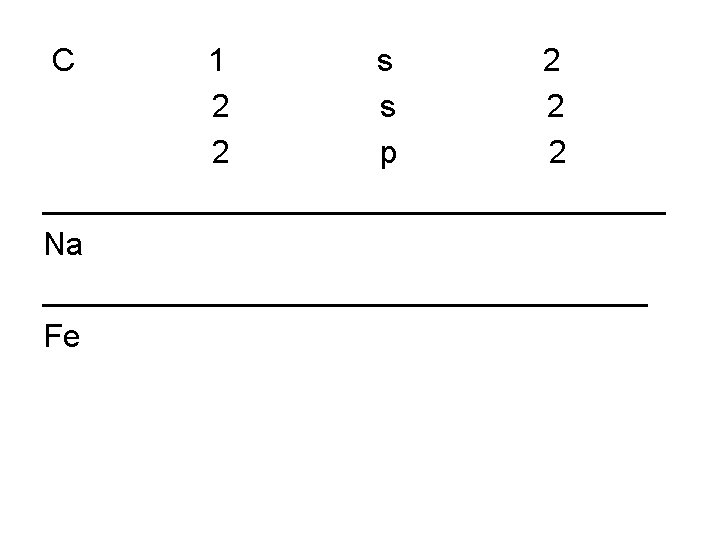

• • • H (1) 1 s 1 He (2) 1 s 2 Li (3) 1 s 1 2 s 2 C ( 6) 1 s 1 2 s 2 2 p 2 Na (11) 1 s 1 2 s 2 2 p 6 3 s 1 Al (13) Fe(26) Ga(31) Ag (47) Nd (60)

• Valence e- determine the ______ proprieties of atoms • Atoms of elements in the same ____ have the same number of valence electrons, so these elements have similar proprieties • These elements are not exactly alike, because atoms have different numbers of ____ in their nuclei and different numbers of _____ in their filled inner energy levels
- Slides: 20