Periodic Table of Elements Ch 5 Sec 2
Periodic Table of Elements • Ch. 5 Sec. 2
Arrangement • Increasing proton # (ATOMIC #) from left to right.

Periods & Groups • Period = horizontal row – 7 periods on table • Group or Family = vertical columns – 18 groups on table
Periods & Groups • Groups (18) • Periods (7)
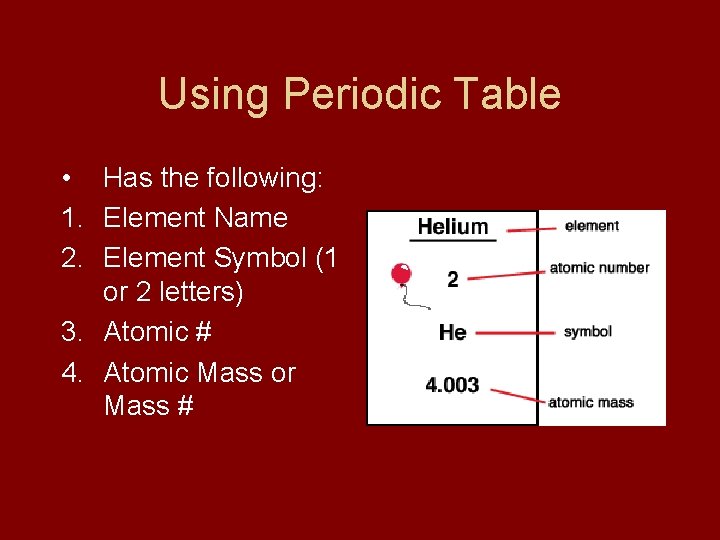
Using Periodic Table • Has the following: 1. Element Name 2. Element Symbol (1 or 2 letters) 3. Atomic # 4. Atomic Mass or Mass #

Atomic # • PROTON # – Helium - 2 p+ • P+ = e • So… Helium also has 2 e-
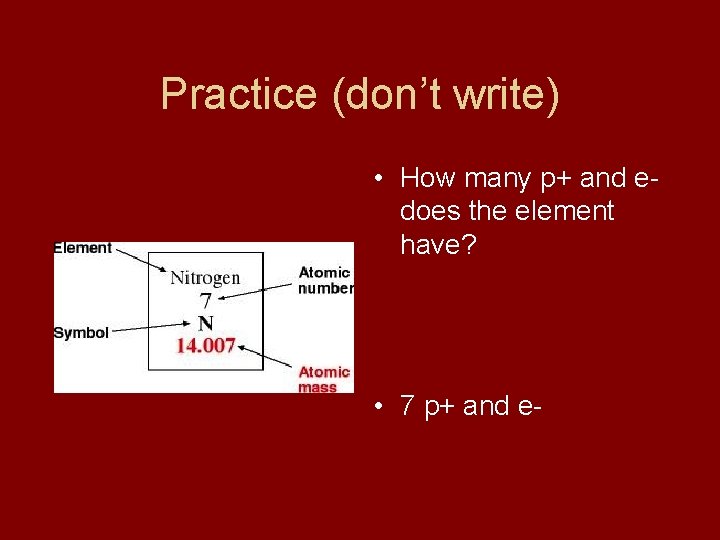
Practice (don’t write) • How many p+ and edoes the element have? • 7 p+ and e-
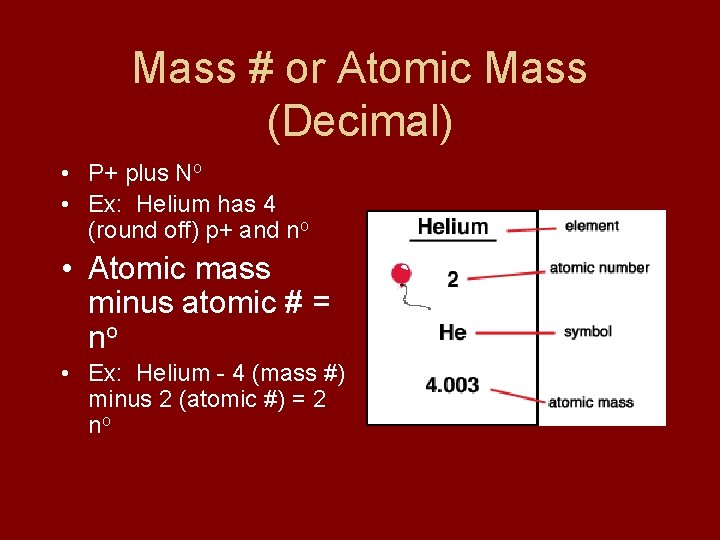
Mass # or Atomic Mass (Decimal) • P+ plus No • Ex: Helium has 4 (round off) p+ and no • Atomic mass minus atomic # = no • Ex: Helium - 4 (mass #) minus 2 (atomic #) = 2 no
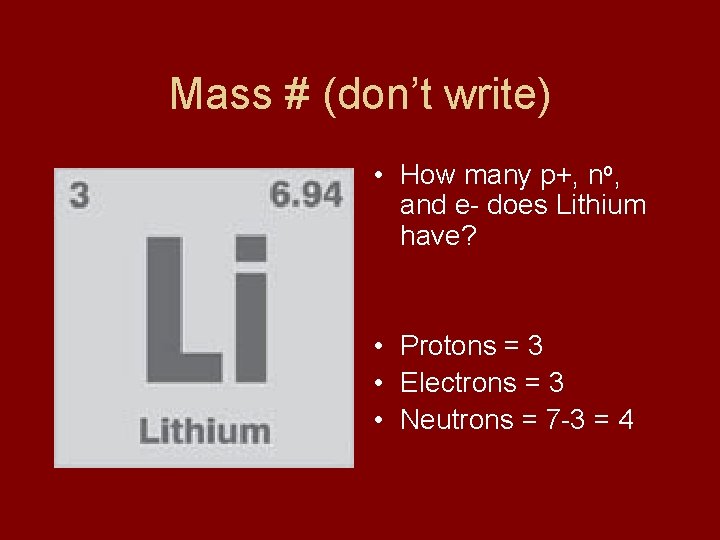
Mass # (don’t write) • How many p+, no, and e- does Lithium have? • Protons = 3 • Electrons = 3 • Neutrons = 7 -3 = 4

Isotopes • • 1. 2. 3. Isotopes = element with diff. # of no Ex: Hydrogen - atomic # =1 (1 proton) H-1 = protium - 0 n H-2 = deuterium - 1 n H-3 = tritium - 2 n
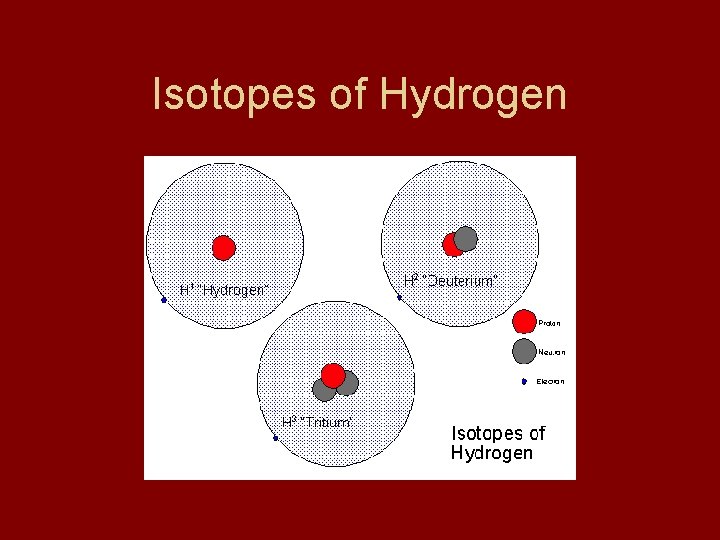
Isotopes of Hydrogen
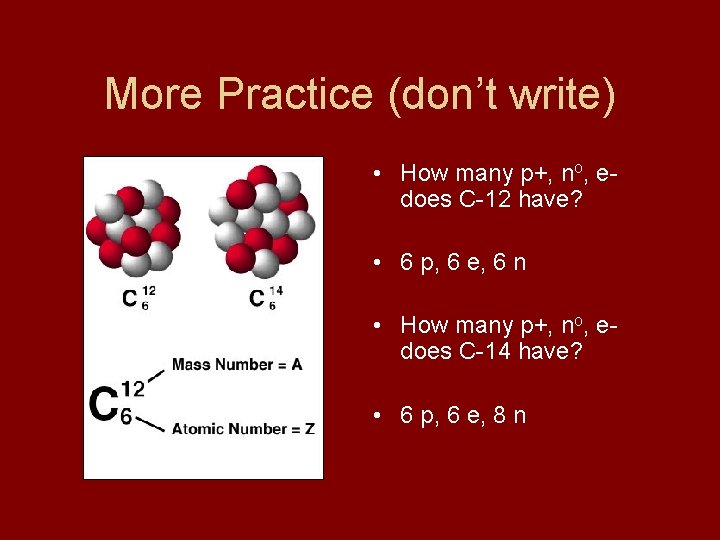
More Practice (don’t write) • How many p+, no, edoes C-12 have? • 6 p, 6 e, 6 n • How many p+, no, edoes C-14 have? • 6 p, 6 e, 8 n
Atomic Mass • Weighted average of all known isotopes (why it’s a decimal) • Ex: Cl-35 (75%) & Cl-37 (25%) on Earth • More Cl-35 so mass on Periodic Table is closer to 35 (35. 45)
Compounds & Ions • Elements swap e- when making compounds • P+ always stay the same

Ions • If outer shells are not full (2 in 1 st shell, 8 in other shells) atom loses or gains e= ion • Not neutral anymore

Cations • Atoms with 1 or 2 e- in outer shell lose e • Cations - lose e (-) and makes atom (+) • *The cat was positive about his loss.
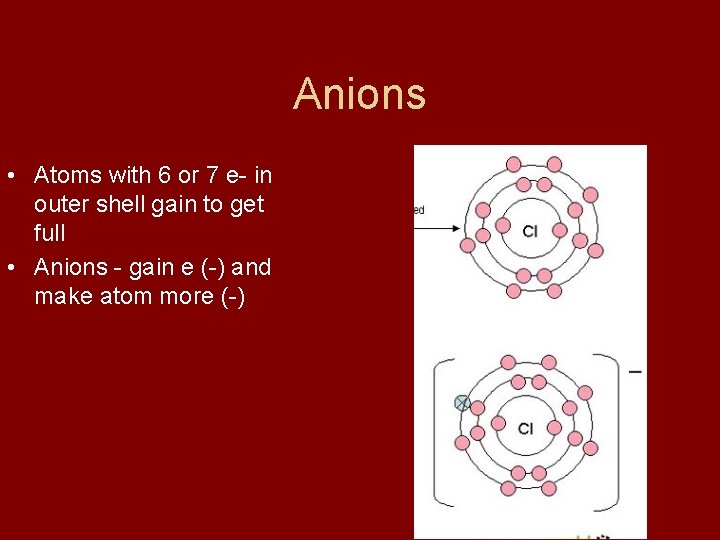
Anions • Atoms with 6 or 7 e- in outer shell gain to get full • Anions - gain e (-) and make atom more (-) Gained Electron

Lithium (don’t write) • Lithium - 1 valence e • Not happy • Gives 1 e- away and has 2 in new outside shell (full) • Cation

Fluorine (don’t write) • Fluorine - 7 valence e • Not happy • Gains 1 e- to get 8 in outer shell (full) • Anion
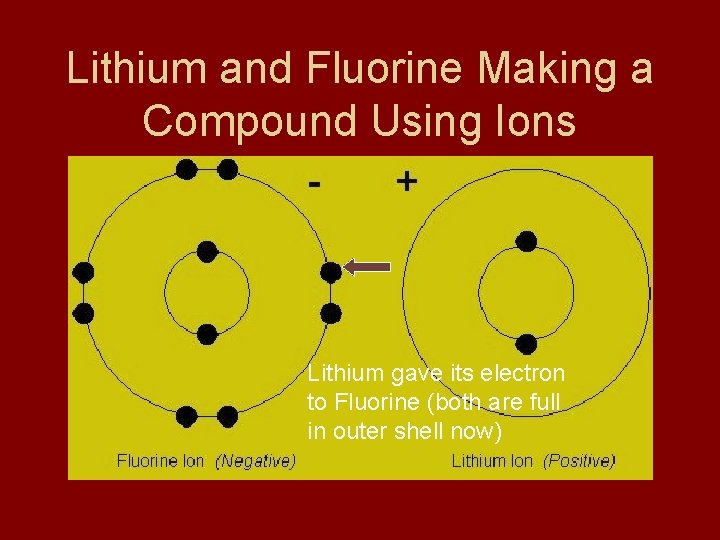
Lithium and Fluorine Making a Compound Using Ions Lithium gave its electron to Fluorine (both are full in outer shell now)
- Slides: 20