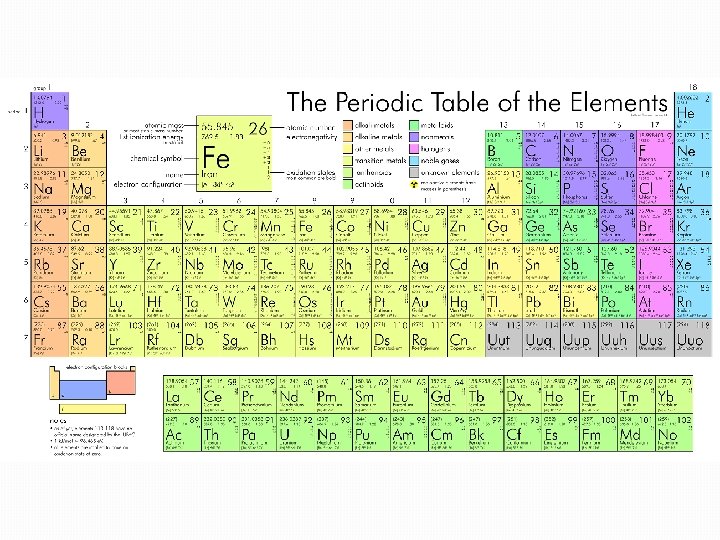
PERIODIC TABLE OF ELEMENTS AND CALCULATIONS Number of

PERIODIC TABLE OF ELEMENTS AND CALCULATIONS
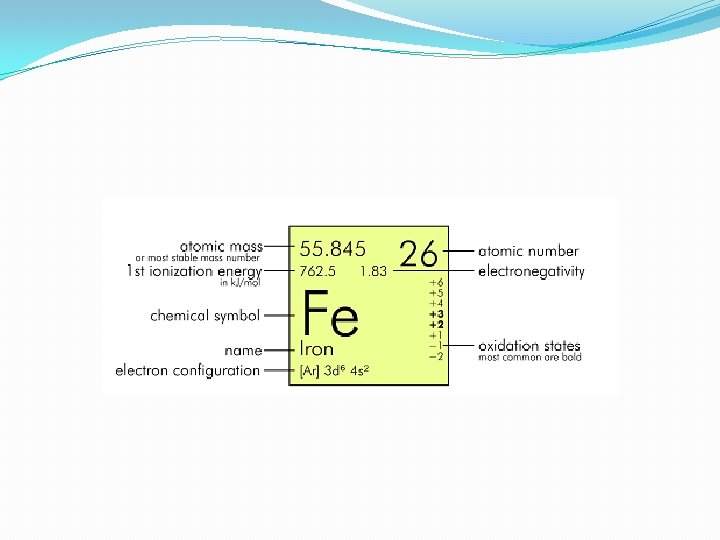
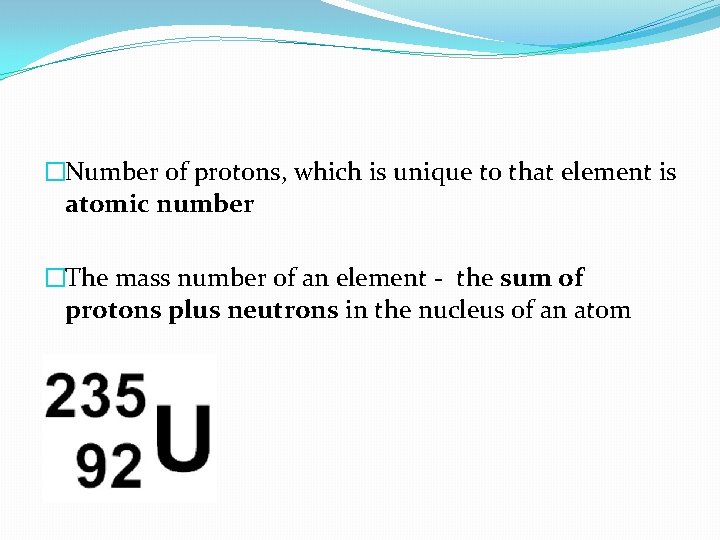
�Number of protons, which is unique to that element is atomic number �The mass number of an element - the sum of protons plus neutrons in the nucleus of an atom

�In chemistry, we calculate and measure the amounts of substances to use in lab �Like in everyday life �Chemical reactions occur everywhere �Simple or complex �All can be written with chemical equations �Reactants and products

�In grocery store we use certain scale for food �Dozen (eggs), case (soda), gross (pencils) ream (paper) q. Example: dozen eggs = 12 eggs

Mole � In chemistry, particles such as atoms, molecules, and ions are counted by the mole, which contains 6. 02 x 1023 items. � This value, known as Avogadro’s number, is a very big number because atoms are so small that it takes an extremely large number of atoms to provide a sufficient amount to weigh and use in chemical reactions. � Avogadro’s number is named for Amedeo Avogadro 1776– 1856, an Italian physicist.

Avogadro’s number tells us that one mole of a compound contains 6. 02 x 1023 of the particular type of particles that make up that compound. � 1 mole of carbon contains 6. 02 x 1023 carbon atoms; � 1 mole of aluminum contains 6. 02 x 1023 aluminum atoms; � 1 mole of sulfur contains 6. 02 x 1023 sulfur atoms
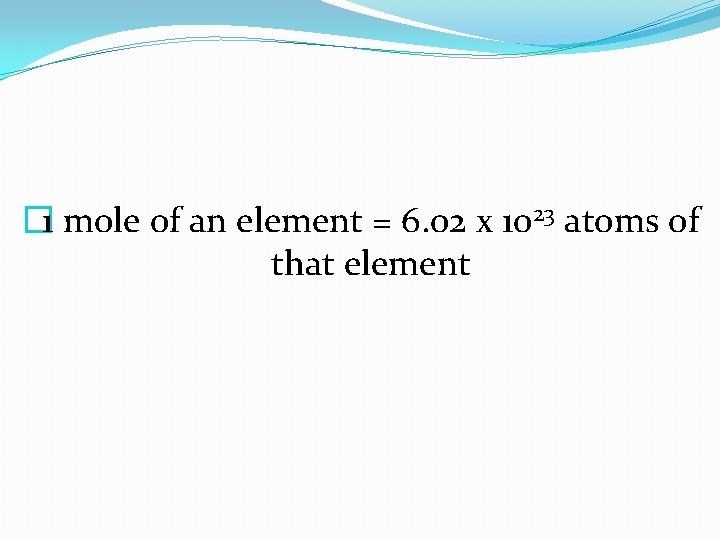
� 1 mole of an element = 6. 02 x 1023 atoms of that element
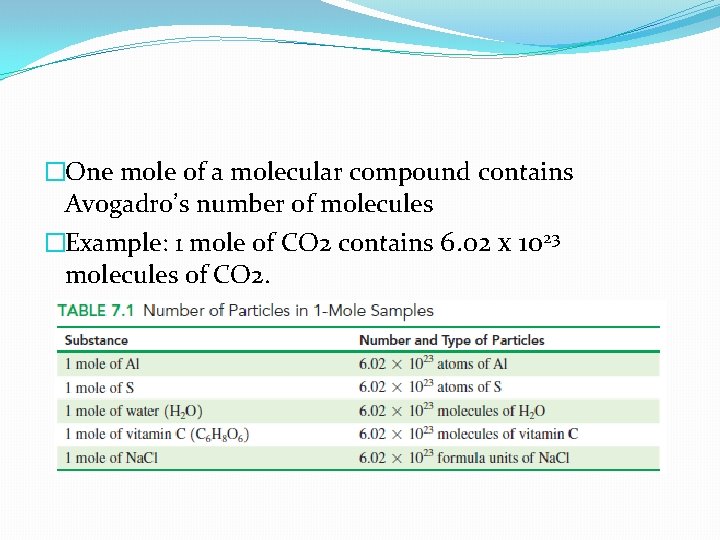
�One mole of a molecular compound contains Avogadro’s number of molecules �Example: 1 mole of CO 2 contains 6. 02 x 1023 molecules of CO 2.
Moles of Elements in a Chemical Formula �Subscripts – indicate the number of atoms of each type of element in the compound q. Example: aspirin (C 9 H 8 O 4) has 9 carbon atoms, 8 hydrogen atoms and 4 oxygen atoms �Also tells the number of moles of each element in 1 mole of aspirin � 9 moles of C atoms, 8 moles of H atoms and 4 moles of O atoms
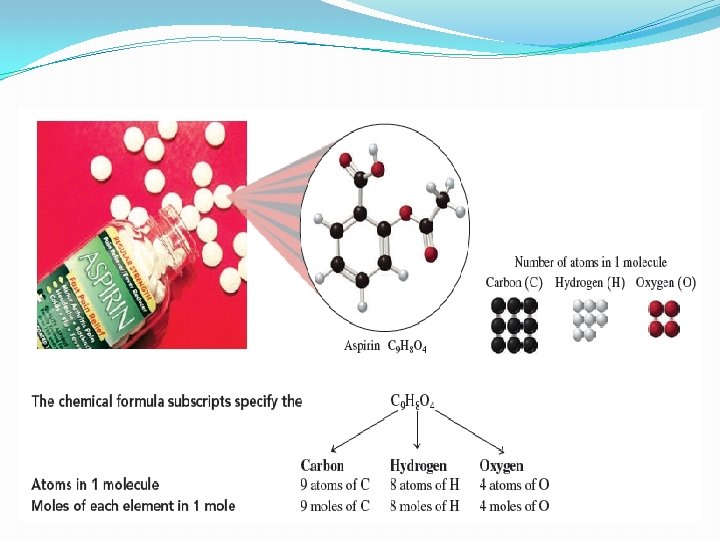
Moles and Elements in a given formula
Molar Mass and Calculations �Single atom or molecule is too small to weigh �It takes a huge number of atoms or molecules to make enough of a substance for you to see �Molar mass – quantity in grams that equals the atomic mass of that element q. Example: carbon has atomic mass of 12. 01 Ø 1 mole of carbon atoms has a mass of 12. 01 g Ø To obtain 1 mole of carbon atoms we must weigh out 12. 01 g of carbon
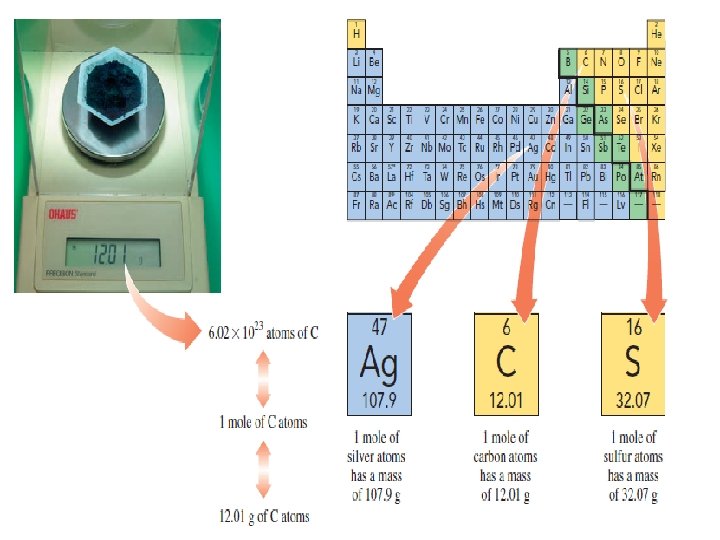
�The molar mass of an element is useful to convert moles of an element to grams, or grams to moles �We can calculate molar mass of a compound

Problem 1 �Li 2 CO 3 �Obtain the molar mass of the compound

Solution 1 � 1. obtain the molar mass of each element: q Li= 6. 941 g/mol q C= 12. 01 g/mol q O= 16 g/mol � 2. Multiply each molar mass by the number of moles (subscript) in the formula � 3. Calculate the molar mass by adding the masses of elements � 4. Solution: 73. 9 g/mol

Problem 2 �Determine the molar mass of the elements: �Pt, U, Mg, Mn, Fe, Zn �C, O, N, H, S �Bi, Po, Xe, Rn, Na

Solution 2 � Pt (195 g/mol) � U (238 g/mol), � Mg (24. 3 g/mol), � Mn (55 g/mol), � Fe (56 g/mol), � Zn (65. 4 g/mol) � C (12 g/mol), � O (16 g/mol), � N (14 g/mol), � H (1 g/mol), � S (32 g/mol) � Bi (209 g/mol), � Po (210 g/mol), � Xe (131. 3 g/mol), � Rn (220 g/mol), � Na (23 g/mol)
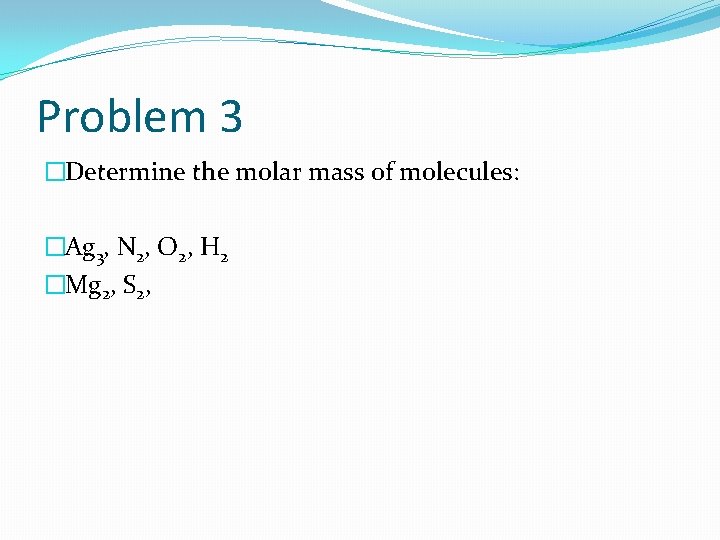
Problem 3 �Determine the molar mass of molecules: �Ag 3, N 2, O 2, H 2 �Mg 2, S 2,

Solution 3 �Ag 2 (2 x 107. 9 g/mol= 215. 8 g/mol), �N 2 (2 x 14 g/mol= 28 g/mol), �O 2 (2 x 16 g/mol= 32 g/mol), �H 2 (2 x 1 g/mol= 2 g/mol) �Mg 2 (2 x 24. 3 g/mol= 48. 6 g/mol), �S 2 (2 x 32 g/mol= 64 g/mol)
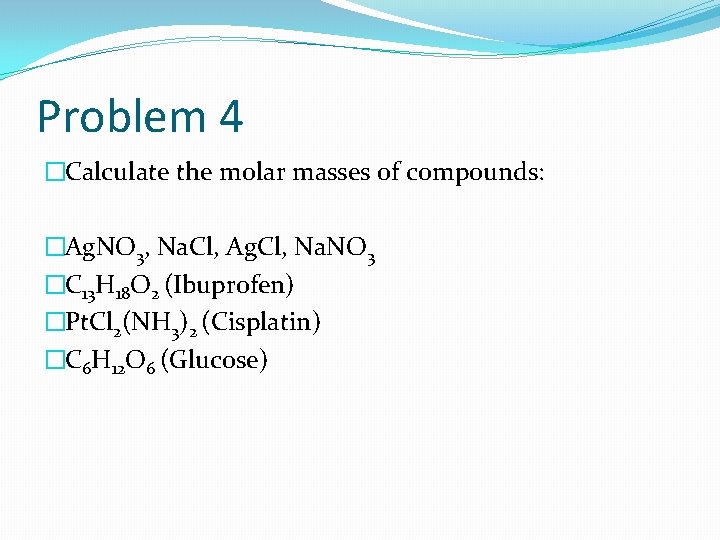
Problem 4 �Calculate the molar masses of compounds: �Ag. NO 3, Na. Cl, Ag. Cl, Na. NO 3 �C 13 H 18 O 2 (Ibuprofen) �Pt. Cl 2(NH 3)2 (Cisplatin) �C 6 H 12 O 6 (Glucose)

Solution 4 �Single work! �Ag. NO 3 = 107. 9 + 14 + 3 x 16= 170 g/mol �Na. Cl= 23 + 35. 4= 58. 4 g/mol �Ag. Cl= 107. 9 + 35. 4= 143. 3 g/mol �Na. NO 3= 23 + 14 + 3 x 16= 85 g/mol �C 13 H 18 O 2= 13 x 12 + 18 x 1 +2 x 16= 206 g/mol �Pt. Cl 2(NH 3)2= 195 + 2 x 35. 4 + (2 x 14 + 6 x 1)= 299. 8 g/mol �C 6 H 12 O 6= 6 x 13 + 12 x 1 + 6 x 16= 186 g/mol

Conclusions �Calculations of molar mass!
Assignments �Calculate the molar mass of nickel (II) hexahydrate (Ni. SO 4 • 6 H 2 O) �Calculate the molar mass of the hydrates formula unit Ni. SO 4 �Calculate the molar mass of PO 4 H 2(CH 2)12 CH 3 �Calculate the molar mass of C 17 H 29 COOH (Linoleic acid) �Calculate the molar mass of C₁₇H₁₉NO₃ (Morphine)
- Slides: 25