Periodic Table Notes Part 1 Created by Harris
Periodic Table Notes Part 1 Created by Harris M. S.
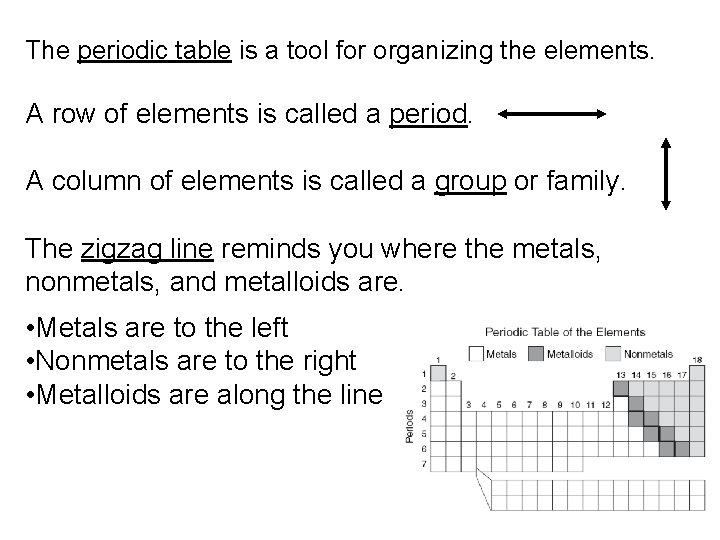
The periodic table is a tool for organizing the elements. A row of elements is called a period. A column of elements is called a group or family. The zigzag line reminds you where the metals, nonmetals, and metalloids are. • Metals are to the left • Nonmetals are to the right • Metalloids are along the line
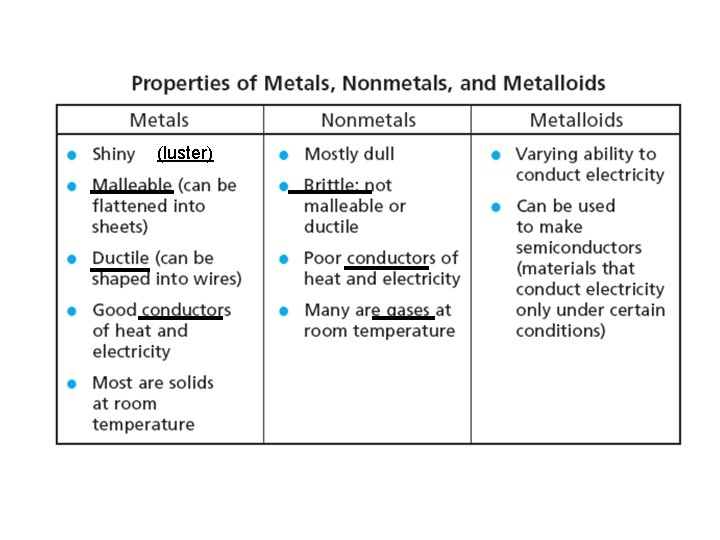
(luster)
Periodic Table Notes Part 2 handout provided
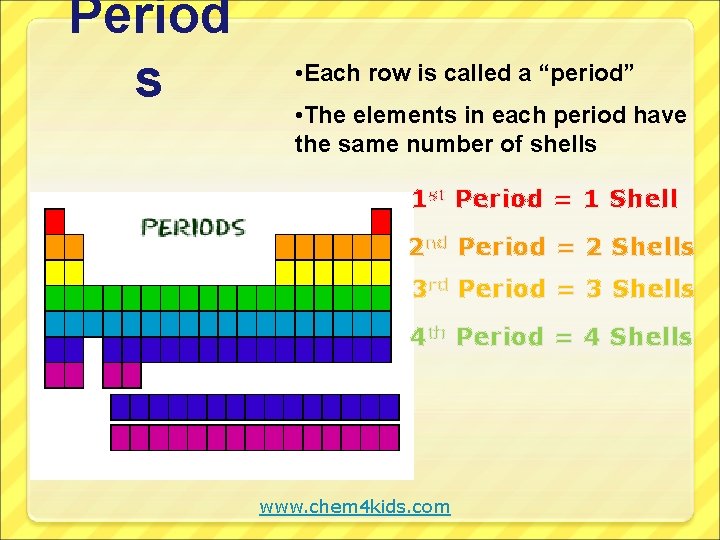
Period s • Each row is called a “period” • The elements in each period have the same number of shells 1 st Period = 1 Shell 2 nd Period = 2 Shells 3 rd Period = 3 Shells 4 th Period = 4 Shells www. chem 4 kids. com
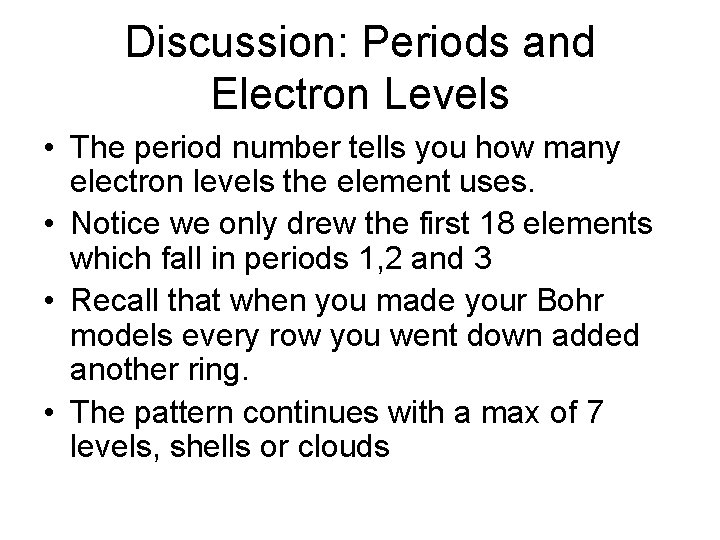
Discussion: Periods and Electron Levels • The period number tells you how many electron levels the element uses. • Notice we only drew the first 18 elements which fall in periods 1, 2 and 3 • Recall that when you made your Bohr models every row you went down added another ring. • The pattern continues with a max of 7 levels, shells or clouds
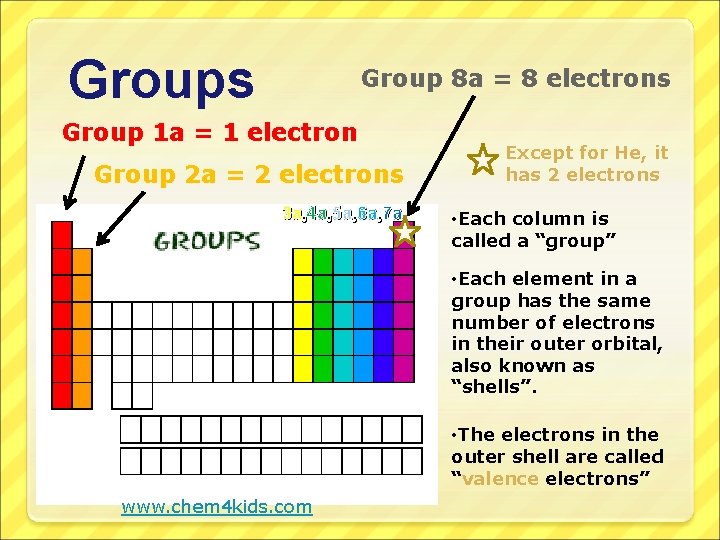
Groups Group 8 a = 8 electrons Group 1 a = 1 electron Group 2 a = 2 electrons 3 a, 4 a, 5 a, 6 a, 7 a Except for He, it has 2 electrons • Each column is called a “group” • Each element in a group has the same number of electrons in their outer orbital, also known as “shells”. • The electrons in the outer shell are called “valence electrons” www. chem 4 kids. com
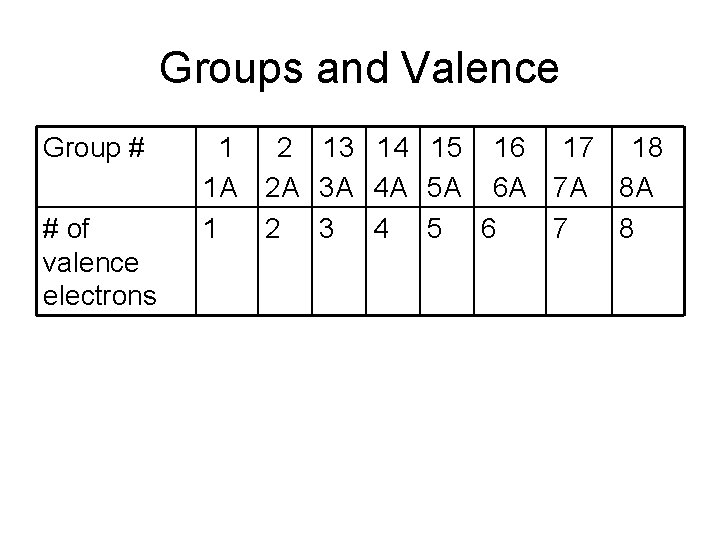
Groups and Valence Group # # of valence electrons 1 1 A 1 2 13 14 15 16 17 18 2 A 3 A 4 A 5 A 6 A 7 A 8 A 2 3 4 5 6 7 8

Discussion: Groups and Valence Electrons • The elements in a group have similar properties and react/behave in similar ways. • Valence electrons are those located in the outermost energy level of an atom – Atoms are “happy” and stable when their outermost energy level is full – all of the Noble gases in group 18 are this way. – Atoms are reactive with other atoms when their outermost energy level is not full. – Atoms in the same group have the same # of valence electrons except the transition metals which do not follow the same pattern – Elements in a family increase in reactivity as you move down.
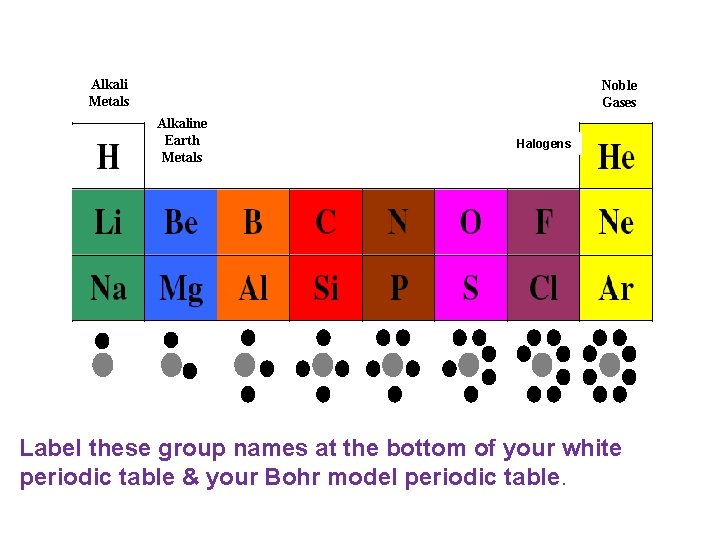
Alkali Metals Noble Gases Alkaline Earth Metals Boron Family Carbon Family Nitrogen Family Oxygen Family Halogens Halides Label these group names at the bottom of your white periodic table & your Bohr model periodic table.

Transition Metals • Transition Metals have slightly different rules for shells and valence electrons. • This is something you will learn about in High School Chemistry. www. chem 4 kids. com

• Write the name of each family at the bottom of the columns on your periodic table using the following information. • Alkali Metals – Most Reactive Metals(Hint: 1 valence electron) • Alkaline Earth Metals – Reactive Metals(Hint: 2 valence electrons) • Halides/Halogens – Most reactive nonmetals (Hint: 7 valence electrons) • Noble Gases – Un reactive non metals (hint: Complete outer shells)
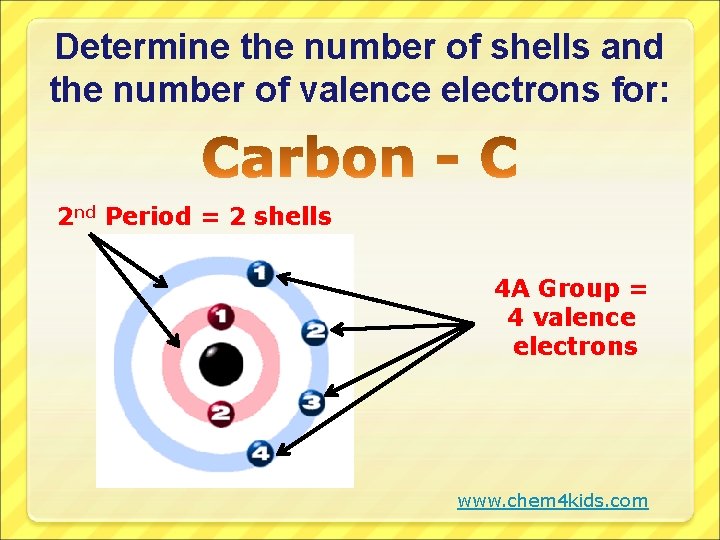
Determine the number of shells and the number of valence electrons for: 2 nd Period = 2 shells 4 A Group = 4 valence electrons www. chem 4 kids. com
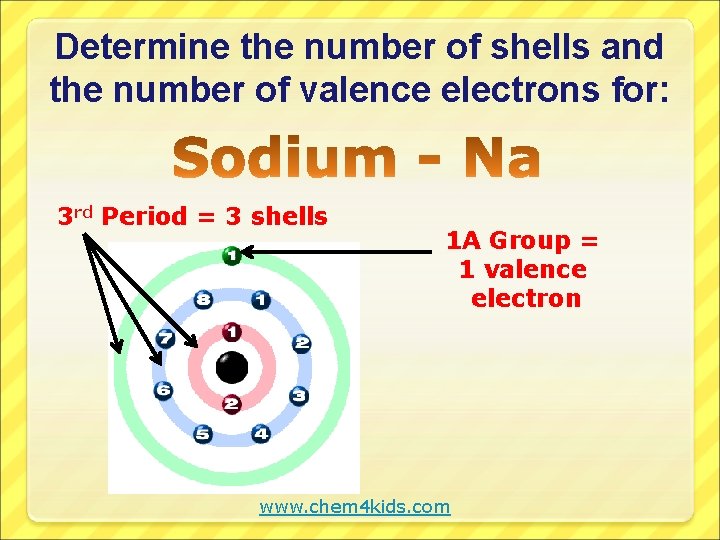
Determine the number of shells and the number of valence electrons for: 3 rd Period = 3 shells 1 A Group = 1 valence electron www. chem 4 kids. com

Write your answers on your handout. Name the element. Number of shells ? Valence electrons ?

Write your answers on your handout. Name the element. Number of shells ? Valence electrons ? Neon 2 nd Period = 2 shells 8 A Group = 8 valence electrons

Write your answers on your handout. Name the element. Number of shells ? Valence electrons ?

Write your answers on your handout. Name the element. Number of shells ? Valence electrons ? Hydrogen 1 st Period = 1 shell 1 A Group = 1 valence electron

Write your answers on your handout. Name the element. Number of shells ? Valence electrons ?

Write your answers on your handout. Name the element. Number of shells ? Valence electrons ? Beryllium 2 nd Period = 2 shells 2 A Group = 2 valence electrons

Write your answers on your handout. Name the element. Number of shells ? Valence electrons ?
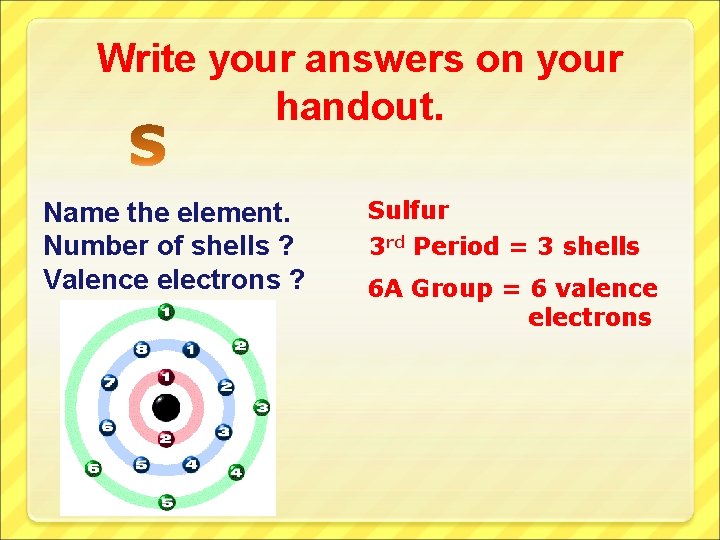
Write your answers on your handout. Name the element. Number of shells ? Valence electrons ? Sulfur 3 rd Period = 3 shells 6 A Group = 6 valence electrons

Write your answers on your handout. Name the element. Number of shells ? Valence electrons ?
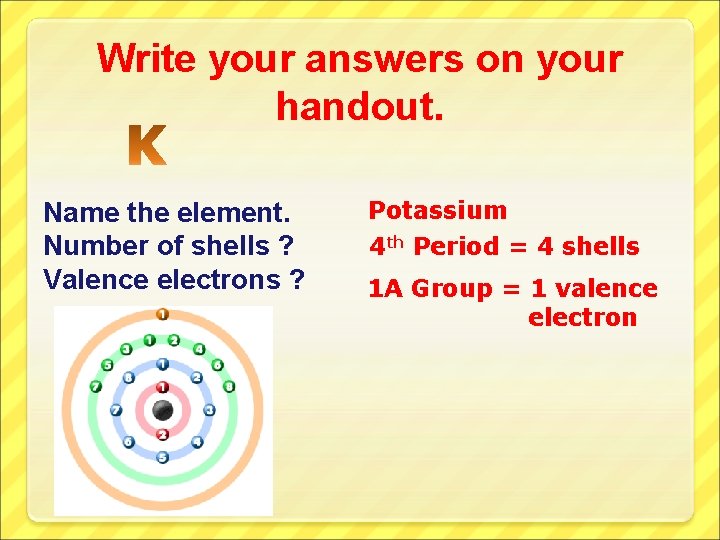
Write your answers on your handout. Name the element. Number of shells ? Valence electrons ? Potassium 4 th Period = 4 shells 1 A Group = 1 valence electron

Write your answers on your handout. Name the element. Number of shells ? Valence electrons ?

Write your answers on your handout. Name the element. Number of shells ? Valence electrons ? Helium 1 st Period = 1 shell 8 A Group = 2 valence electrons • Helium is the exception in Group 8 a. • Since it has just one shell, that shell can only fit 2 electrons instead of 8. • It is in this group because all the elements have a full outer shell.

End of Study Guide
- Slides: 27