Periodic Table Notes Part 1 8 th Grade
Periodic Table Notes – Part 1 8 th Grade Science 2017 -2018 Schindler
Periodic Table Rap – My version Here’s a little story I like to tell… About a few elements I know so well. I’ve got metals on my left. Nonmetals to my right. But metalloids like to take this flight. They go: Boron, Silicon, Down, Across – WHAT? ! Down, Across. https: //www. youtube. com/watch? v=5 t. Tbxf_w. T 6 k
Periodic Table Song & Dance! The Groups on the table go up and down. Up and down. The Groups on the table go up and down. Groups are where like elements can be found. The Periods on the table go left to right. Left to right. The Periods on the table go left to right. Elements aren’t alike going left to right. https: //www. youtube. com/watch? v=JBWDm. Hidz. Jc

Periodic Table Song & Dance! https: //www. youtube. com/watch? v=_4 -P 2 IZm 1 g 8 http: //www. youtube. com/watch? v=r. SAai. YKF 0 cs
History of Periodic Table • History of Periodic Table: – Dimitri Mendeleev’s Periodic Table (1860’s): • He noticed patterns appeared when elements were arranged in order of increasing atomic mass • He also noticed that when he moved elements into groups where they fit best, there were blank spaces left on his table • He concluded that these were spaces for elements not yet discovered – Modern Periodic Table (early 1900’s to present): • The current Periodic Table is arranged in order of increasing atomic number (# of protons)

History of Periodic Table
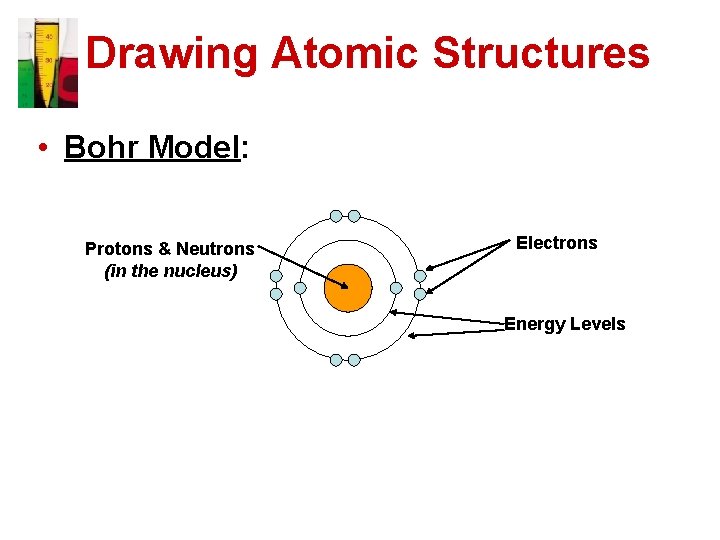
Drawing Atomic Structures • Bohr Model: Protons & Neutrons (in the nucleus) Electrons Energy Levels
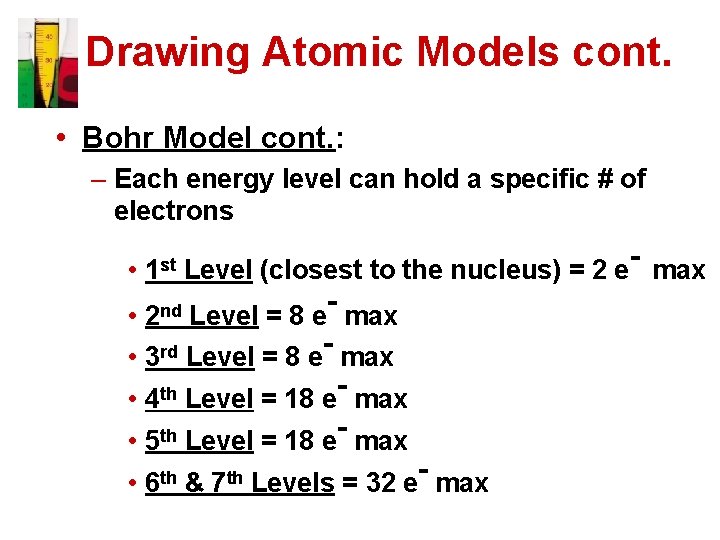
Drawing Atomic Models cont. • Bohr Model cont. : – Each energy level can hold a specific # of electrons • 1 st Level (closest to the nucleus) = 2 e- max • Level = 8 e max • 3 rd Level = 8 e- max th • 4 Level = 18 e max • 5 th Level = 18 e- max 2 nd • 6 th & 7 th Levels = 32 e- max
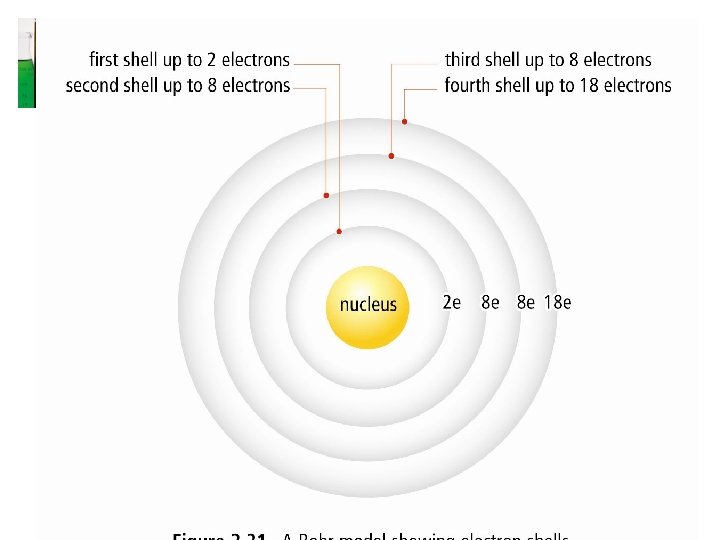
Energy Levels / Orbitals

Bohr Model Practice Lab Follow the instructions on your card to create Bohr models for the elements on the following slides.
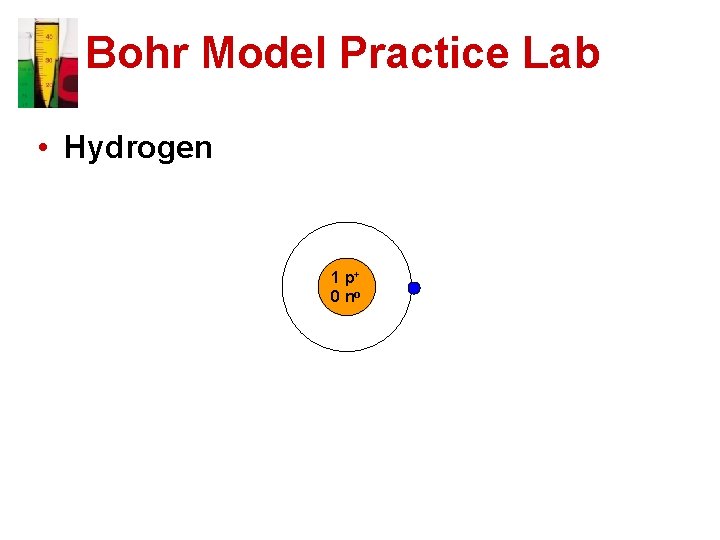
Bohr Model Practice Lab • Hydrogen 1 p+ 0 no
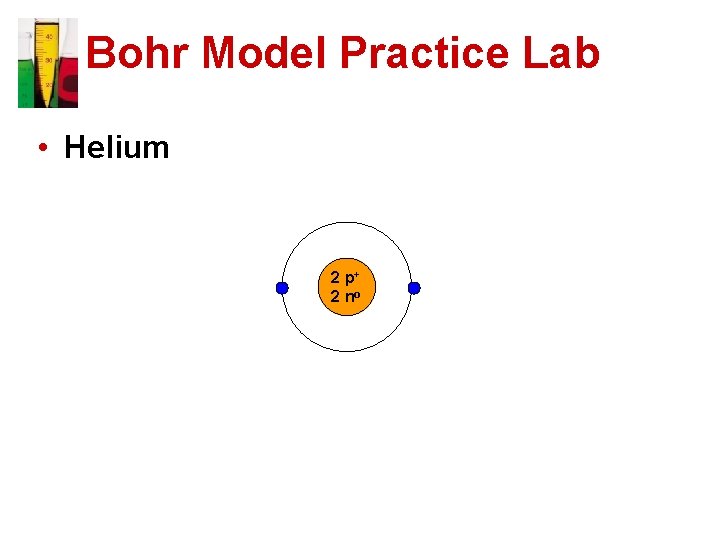
Bohr Model Practice Lab • Helium 2 p+ 2 no
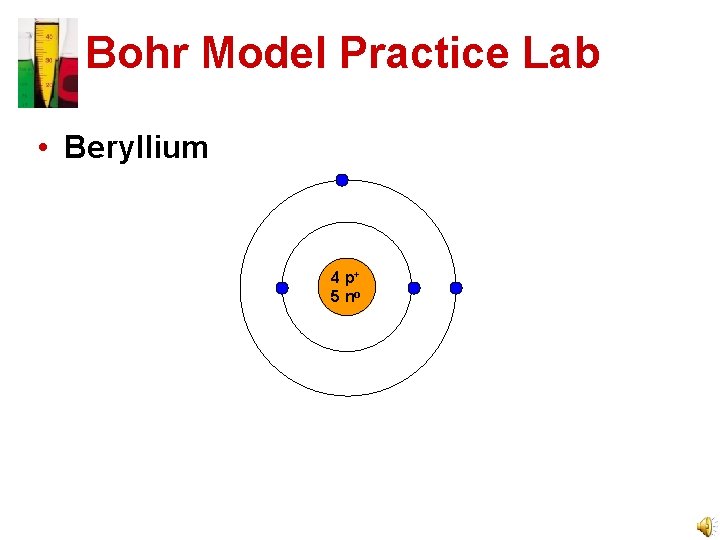
Bohr Model Practice Lab • Beryllium 4 p+ 5 no
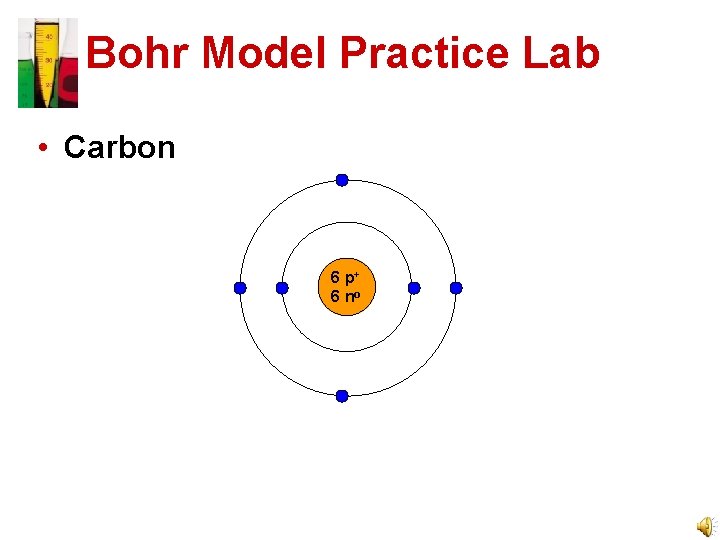
Bohr Model Practice Lab • Carbon 6 p+ 6 no
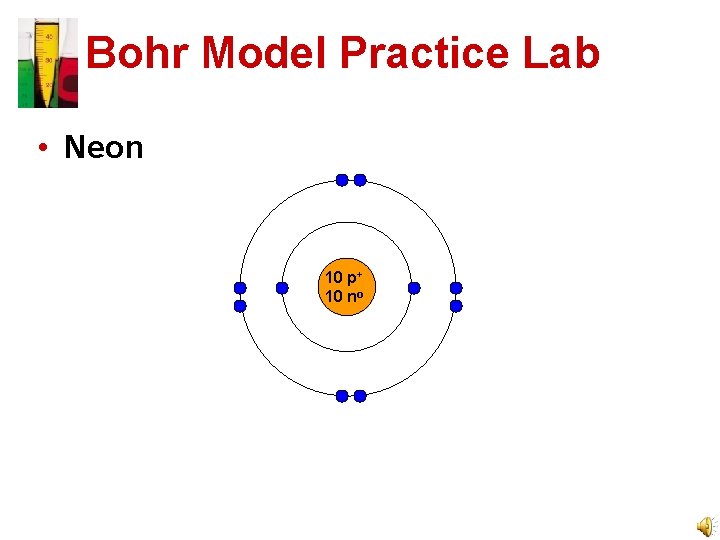
Bohr Model Practice Lab • Neon 10 p+ 10 no
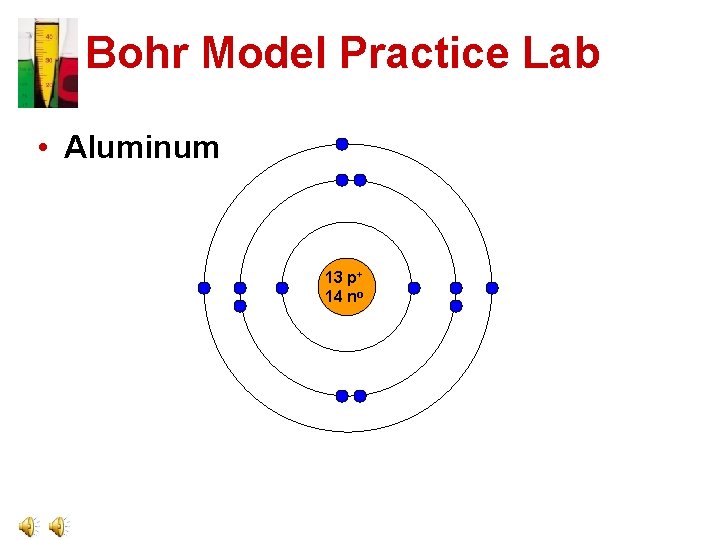
Bohr Model Practice Lab • Aluminum 13 p+ 14 no
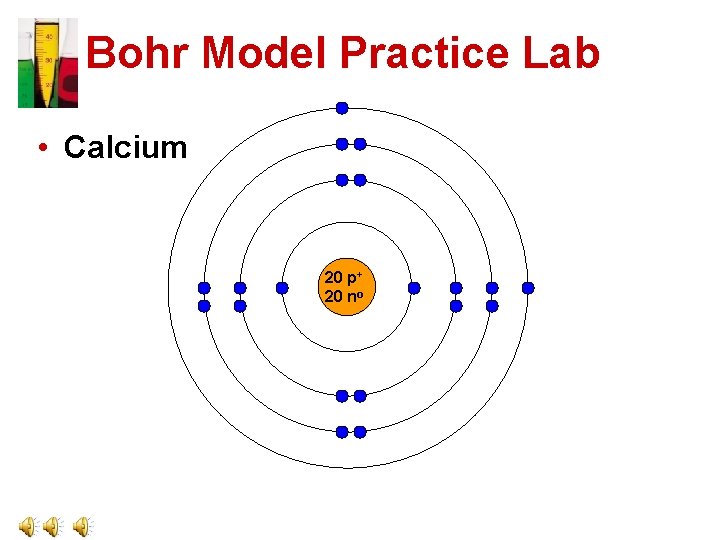
Bohr Model Practice Lab • Calcium 20 p+ 20 no

Valence Electrons • Valence Electrons = electrons located in the outermost energy level of an atom – These are VERY important because they tell us how an element will react with other elements/substances (they are involved with BONDING!) – Valence electrons have the most energy
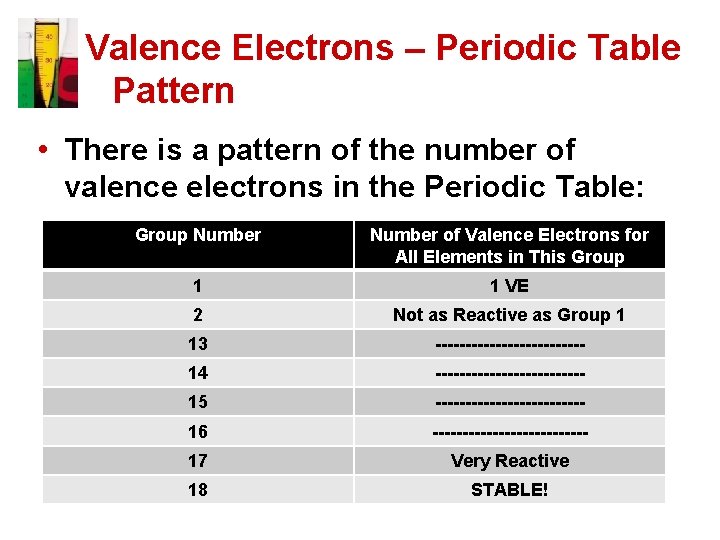
Valence Electrons – Periodic Table Pattern • There is a pattern of the number of valence electrons in the Periodic Table: Group Number of Valence Electrons for All Elements in This Group 1 1 VE 2 Not as Reactive as Group 1 13 ------------- 14 ------------- 15 ------------- 16 ------------- 17 Very Reactive 18 STABLE!
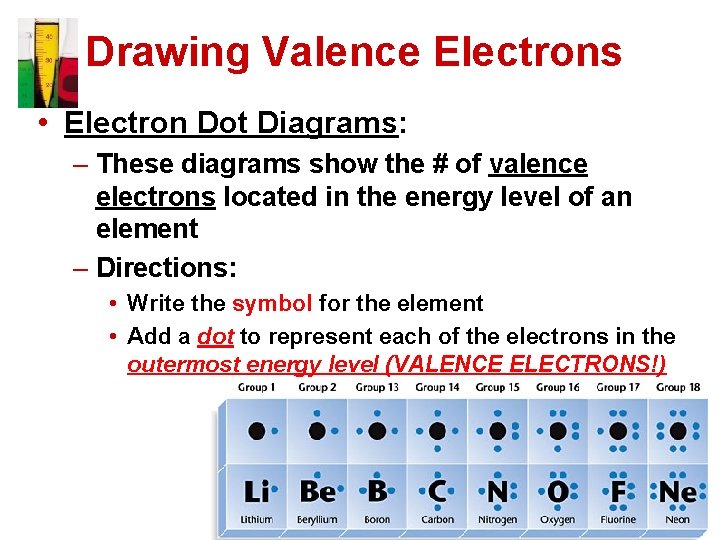
Drawing Valence Electrons • Electron Dot Diagrams: – These diagrams show the # of valence electrons located in the energy level of an element – Directions: • Write the symbol for the element • Add a dot to represent each of the electrons in the outermost energy level (VALENCE ELECTRONS!)
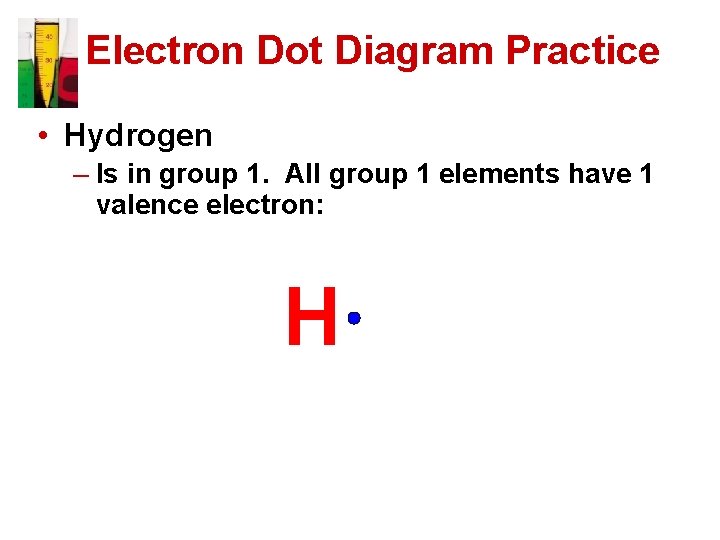
Electron Dot Diagram Practice • Hydrogen – Is in group 1. All group 1 elements have 1 valence electron: H
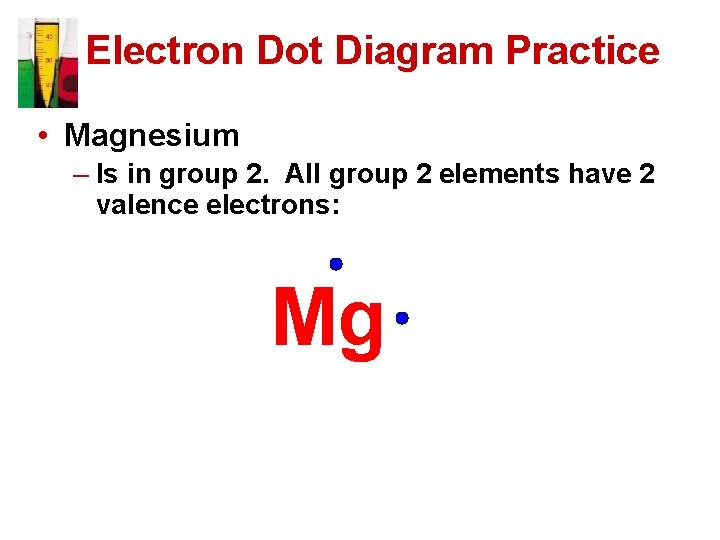
Electron Dot Diagram Practice • Magnesium – Is in group 2. All group 2 elements have 2 valence electrons: Mg
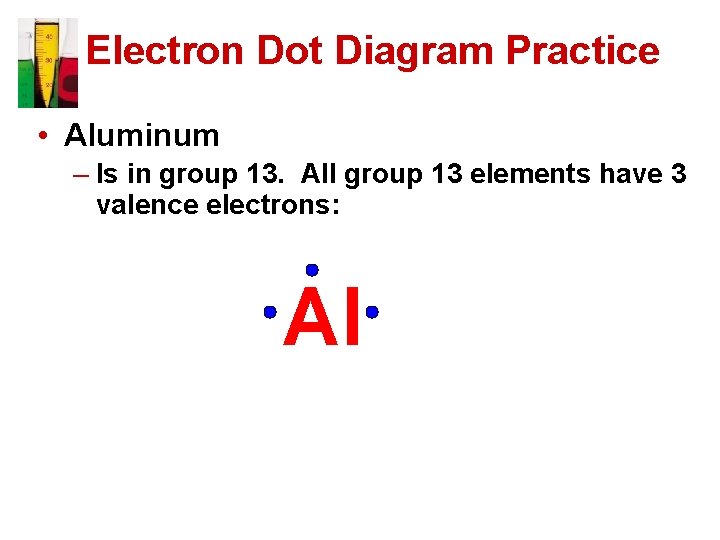
Electron Dot Diagram Practice • Aluminum – Is in group 13. All group 13 elements have 3 valence electrons: Al
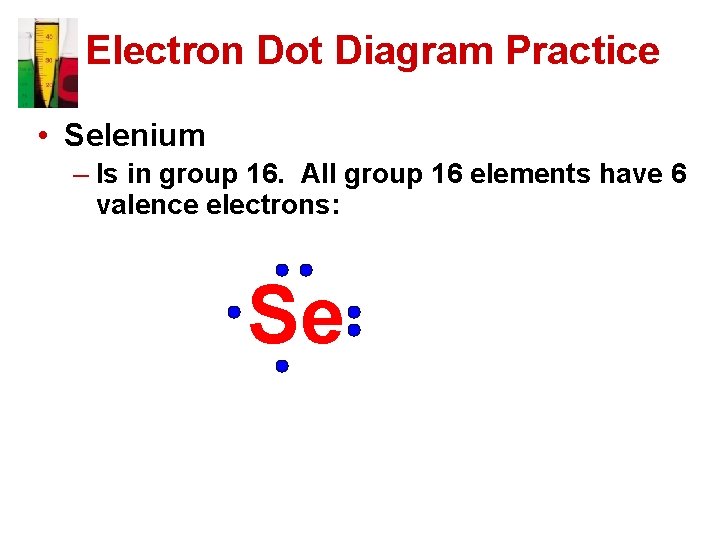
Electron Dot Diagram Practice • Selenium – Is in group 16. All group 16 elements have 6 valence electrons: Se

Electron Dot Diagram Practice • Helium – Is in group 18. All group 18 elements have 8 valence electrons EXCEPT for Helium only has 2: He Helium is the EXCEPTION in group 18!
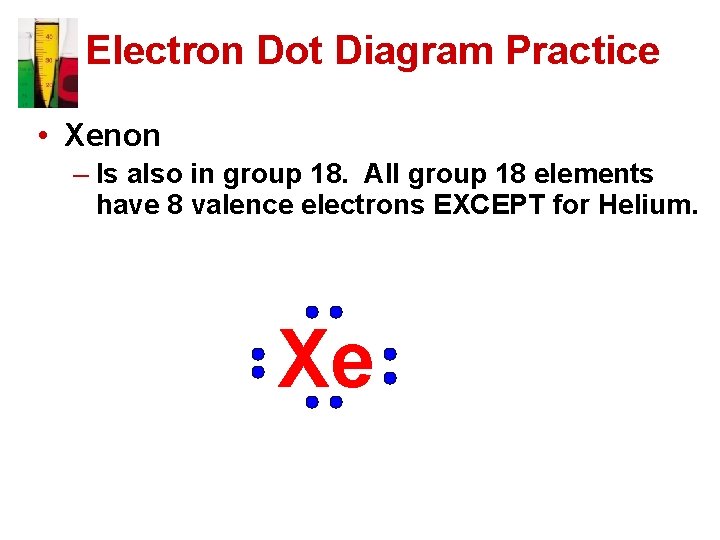
Electron Dot Diagram Practice • Xenon – Is also in group 18. All group 18 elements have 8 valence electrons EXCEPT for Helium. Xe

Valence Electrons - Octet Rule • OCTET RULE – Atoms desire to have a full outer energy level of electrons (they want a complete octet) – A full outermost energy level of electrons = stability!!! • EVERYONE IS JEALOUS OF THE NOBLE GASES - THEY ARE SO STABLE AND HAVE A FULL OCTET ALREADY! They’re NOBLE!!!!!!! Atoms on the LEFT side of the Periodic Table tend to LOSE their valence electrons to get to a full outer energy level. Atoms on the RIGHT side of the Periodic Table tend to GAIN their valence electrons to get to a full outer energy level.
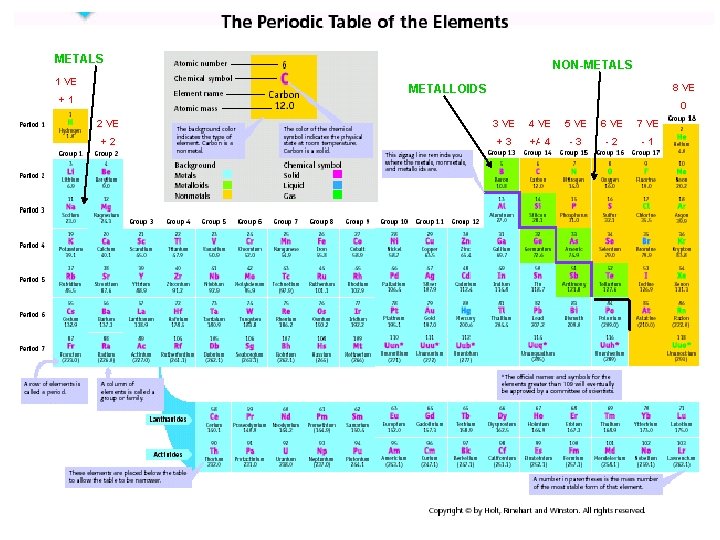
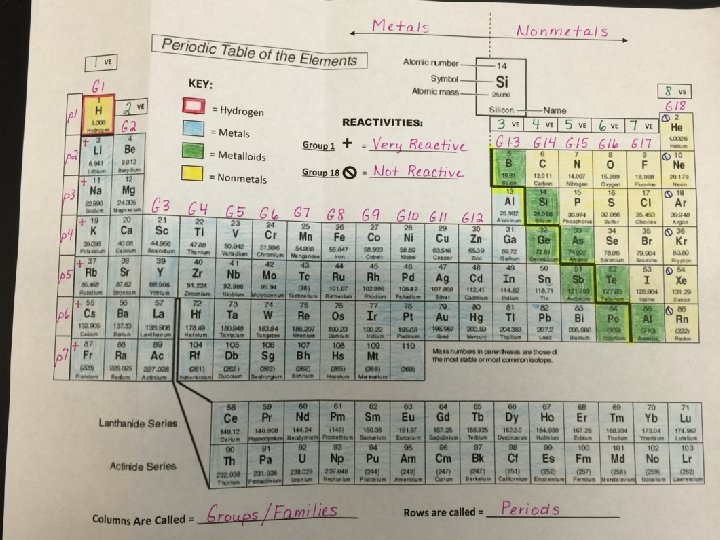
Periodic Table METALS 1 VE NON-METALS 8 VE METALLOIDS +1 0 2 VE 3 VE 4 VE 5 VE 6 VE 7 VE +2 +3 +/- 4 -3 -2 -1
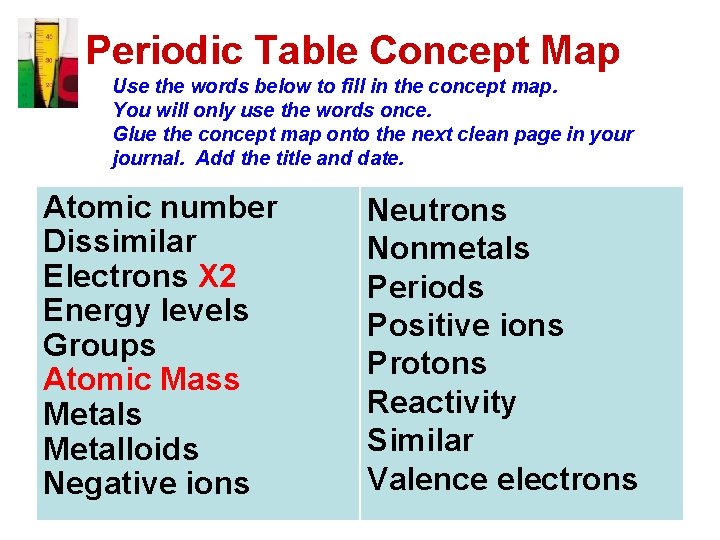
Periodic Table Concept Map Use the words below to fill in the concept map. You will only use the words once. Glue the concept map onto the next clean page in your journal. Add the title and date. Atomic number Dissimilar Electrons X 2 Energy levels Groups Atomic Mass Metalloids Negative ions Neutrons Nonmetals Periods Positive ions Protons Reactivity Similar Valence electrons
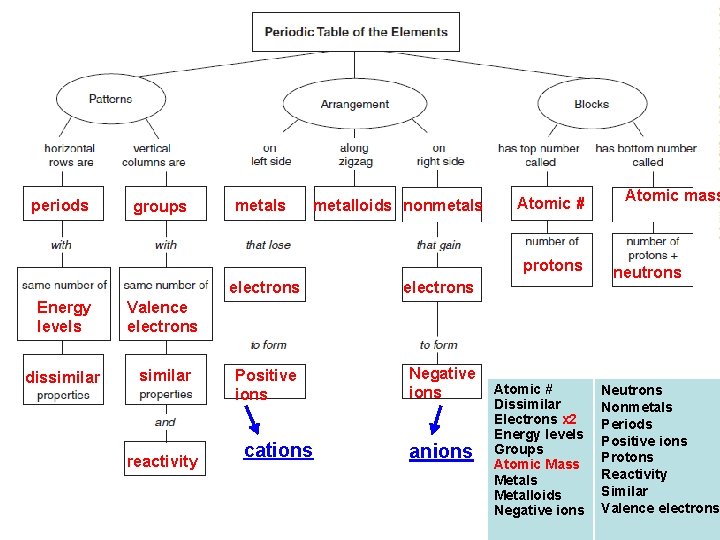
periods groups metalloids nonmetals Atomic # protons Energy levels Valence electrons dissimilar reactivity electrons Positive ions Negative ions cations anions Atomic # Dissimilar Electrons x 2 Energy levels Groups Atomic Mass Metalloids Negative ions Atomic mass neutrons Nonmetals Periods Positive ions Protons Reactivity Similar Valence electrons
Periodic Table Notes – Part 2 8 th Grade Science

Groups / Families • The up-&-down columns in the Periodic Table are called groups / families • Elements in a group / family have similar properties • These similar properties occur because elements in a group / family have the same # of valence electrons • Think of your family having similar properties – so do elements in a family
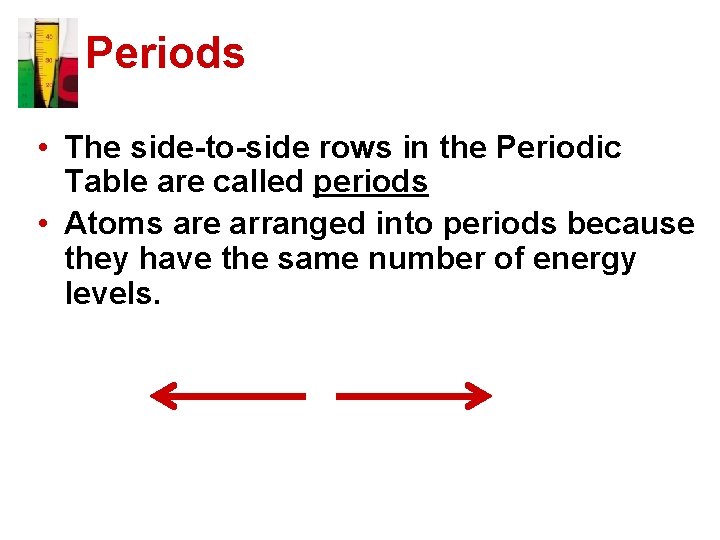
Periods • The side-to-side rows in the Periodic Table are called periods • Atoms are arranged into periods because they have the same number of energy levels.

Symbols / Names of Elements – Don’t Write This! • Some of the elements have symbols very different than their names. • This is because the original name of the element was in another language and may have a Latin or Greek origin. • Examples: – potassium, K, is kalium in Latin – sodium, Na, is natrium in Latin • Others are named because of different reasons. – Ex: Tungsten was first called Wolfram (thus the symbol W) because of minerals in which it was found. Eventually it was renamed Tungsten but kept the Wolfram symbol.
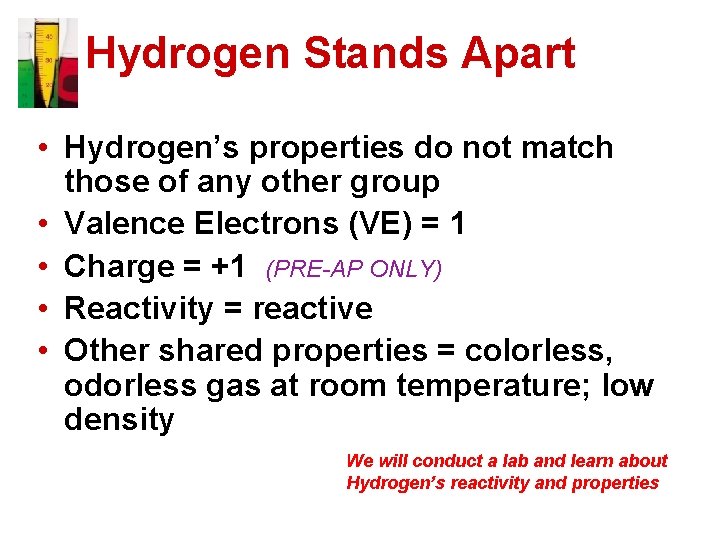
Hydrogen Stands Apart • Hydrogen’s properties do not match those of any other group • Valence Electrons (VE) = 1 • Charge = +1 (PRE-AP ONLY) • Reactivity = reactive • Other shared properties = colorless, odorless gas at room temperature; low density We will conduct a lab and learn about Hydrogen’s reactivity and properties

Group 1: Alkali Metals • • Valence Electrons (VE) = 1 Charge = +1 (PRE-AP ONLY) Reactivity = very reactive Other shared properties = soft; silver colored; shiny; low density Alkali Metals Characteristics & Reacting in Water Brainiacs Alkali Metals

Group 2: Alkali-Earth Metals • Valence Electrons (VE) = 2 • Charge = +2 (PRE-AP ONLY) • Reactivity = very reactive, but less reactive than alkali metals • Other shared properties = silver colored; more dense than alkali metals Alkali-Earth Metals Reacting in Water

Groups 3 -12: Transition Metals • • Metals Valence Electrons (VE) = 1 or 2 Charge = +1, +2 (PRE-AP ONLY) Reactivity = less reactive than alkali-earth metals • Other shared properties = shiny; good conductors of thermal energy and electric current; higher densities and melting points (except mercury) than groups 1 & 2 elements

Group 13: Boron Group • • Valence Electrons (VE) = 3 Charge = +3 (PRE-AP ONLY) Reactivity = reactive Other shared properties = solid at room temperature
Group 14: Carbon Group • • Valence Electrons (VE) = 4 Charge = +/- 4 (PRE-AP ONLY) Reactivity = varies Other shared properties = solid at room temperature

Group 15: Nitrogen Group • • Valence Electrons (VE) = 5 Charge = -3 (PRE-AP ONLY) Reactivity = varies Other shared properties = all but nitrogen are solid at room temperature

Group 16: Oxygen Group • • Valence Electrons (VE) = 6 Charge = -2 (PRE-AP ONLY) Reactivity = reactive Other shared properties = all but oxygen are solid at room temperature

Group 17: Halogens • • Valence Electrons (VE) = 7 Charge = -1 (PRE-AP ONLY) Reactivity = very reactive Other shared properties = poor conductors of electric current; react violently with alkali metals to form salts; never found uncombined in nature Halogens

Group 18: Noble Gases • Valence Electrons (VE) = 8 (2 for Helium) – Have a full outermost energy level. They do not want to gain or lose e- (this is why they are stable). • Charge = 0 (PRE-AP ONLY) • Reactivity = unreactive • Other shared properties = colorless, odorless gases at room temperature Helium Myth. Busters Helium

Nobles gases glow different colors when electricity is passed through them…

- Slides: 47