Periodic Table n n n Electron configurations Charges
Periodic Table n n n Electron configurations Charges (stable ions for compounds) Trends (size, ionization energy, electronegativity) Polarity of bonds (greater difference in electronegativity, more polar) Determine formulas of compounds (Ionic and Non-ionic)
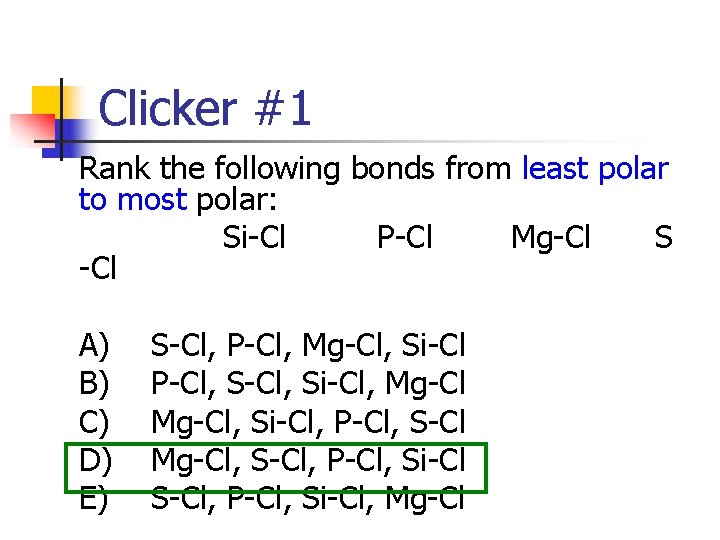
Clicker #1 Rank the following bonds from least polar to most polar: Si-Cl P-Cl Mg-Cl S -Cl A) B) C) D) E) S-Cl, P-Cl, Mg-Cl, Si-Cl P-Cl, Si-Cl, Mg-Cl, Si-Cl, P-Cl, S-Cl Mg-Cl, S-Cl, P-Cl, Si-Cl, Mg-Cl

Localized Electron Model (LEM) n n Increase probability that we know where electrons are We assume electrons are always where we place them
Localized Electron Model (LEM) n n Increase probability that we know where electrons are We assume electrons are always where we place them FORMULA H 2 0 SHAPES PROPERTIES OF MOLECULES
Localized Electron Model (LEM) n n Increase probability that we know where electrons are We assume electrons are always where we place them FORMULA SHAPES PROPERTIES OF MOLECULES H 2 0 n Electrons shared in order to lower energy state of molecule (“fill shell”)

Localized Electron Model (LEM) n n n LEM = LEWIS STRUCTURES!! Assume ONLY the valence electrons matter I. e. - N : 1 s 22 p 3
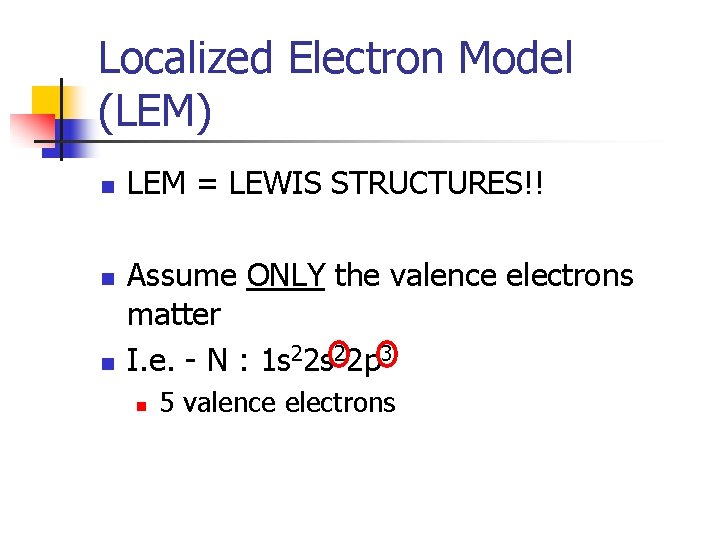
Localized Electron Model (LEM) n n n LEM = LEWIS STRUCTURES!! Assume ONLY the valence electrons matter I. e. - N : 1 s 22 p 3 n 5 valence electrons

Clicker #2 How many electrons would hydrogen have to share in a covalent bond in order to have a noble gas electron configuration? A) B) C) D) E) 0 2 3 6 8

Clicker #3 How many valence electrons are in NO 3–? A) B) C) D) E) 4 18 23 24 32
- Slides: 9