Periodic Table Mr Fleming Periodic Table Development n

Periodic Table Mr. Fleming
Periodic Table Development n In 1869, Russian chemist Dmitri Mendeleev organized elements into a table based on atomic mass and similar properties. n Mendeleev stated that the properties of elements are a periodic function of their atomic masses.
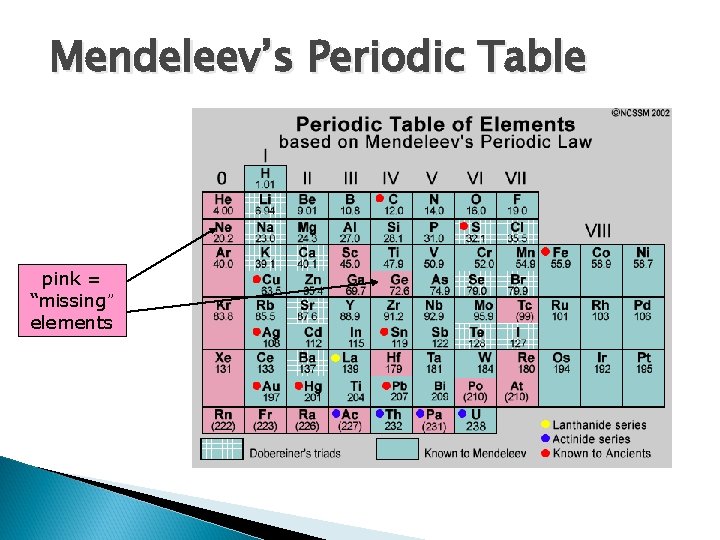
Mendeleev’s Periodic Table pink = “missing” elements

Mendeleev’s Prediction Mendeleev’s table had several missing elements. When these elements were discovered, they were almost exactly as Mendeleev predicted. n The following is an example of an element that Mendeleev predicted, and we now know as the element Germanium… n
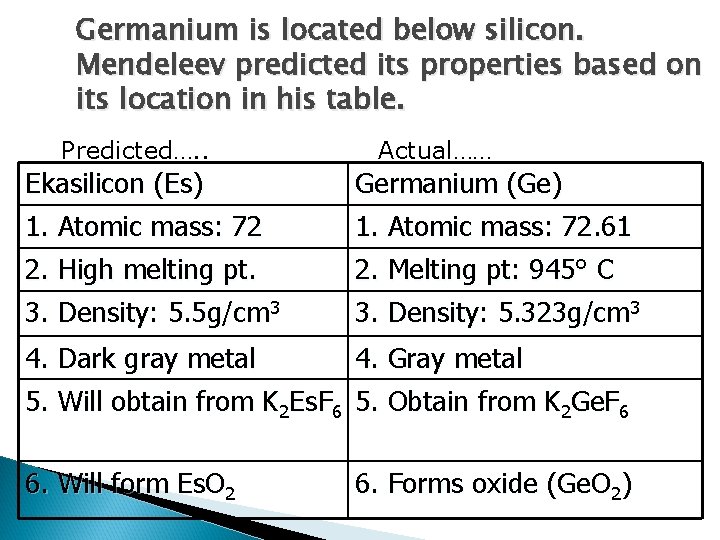
Germanium is located below silicon. Mendeleev predicted its properties based on its location in his table. Predicted…. . Actual…… Ekasilicon (Es) 1. Atomic mass: 72 Germanium (Ge) 1. Atomic mass: 72. 61 2. High melting pt. 3. Density: 5. 5 g/cm 3 2. Melting pt: 945° C 3. Density: 5. 323 g/cm 3 4. Dark gray metal 4. Gray metal 5. Will obtain from K 2 Es. F 6 5. Obtain from K 2 Ge. F 6 6. Will form Es. O 2 6. Forms oxide (Ge. O 2)
Modern Periodic Law Henry Moseley revised Mendeleev’s periodic table by using atomic number (rather than atomic mass) to organize the elements. n Atomic number is the basis for our current periodic table. n

Alternative Periodic Tables Dalton’s Periodic Table
Organization of the Periodic Table: n Rows on the periodic table are called PERIODS n Columns on the periodic table are called GROUPS or FAMILIES
Periodic Table Organization There are 7 periods and 18 groups. n Elements with similar electron arrangements are placed in the same group. n Elements in groups are also listed in order of their increasing electron energy levels (shells). n The properties of elements are determined by their electron arrangements. Therefore, elements in the same group have similar chemical behavior. n

Periodic Trends A periodic trend is a repeating pattern of properties. n We will focus on five trends: 1. size of atom 2. metallic character 3. number of valence electrons 4. predicted charge on ions 5. reactivity n
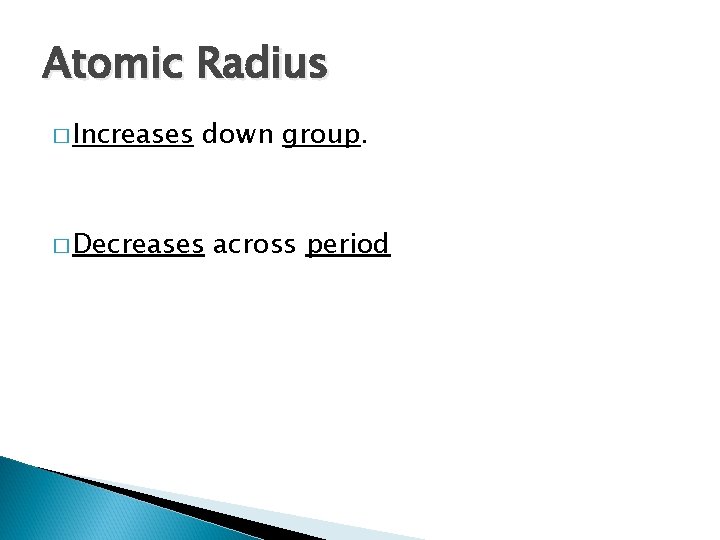
Atomic Radius � Increases down group. � Decreases across period
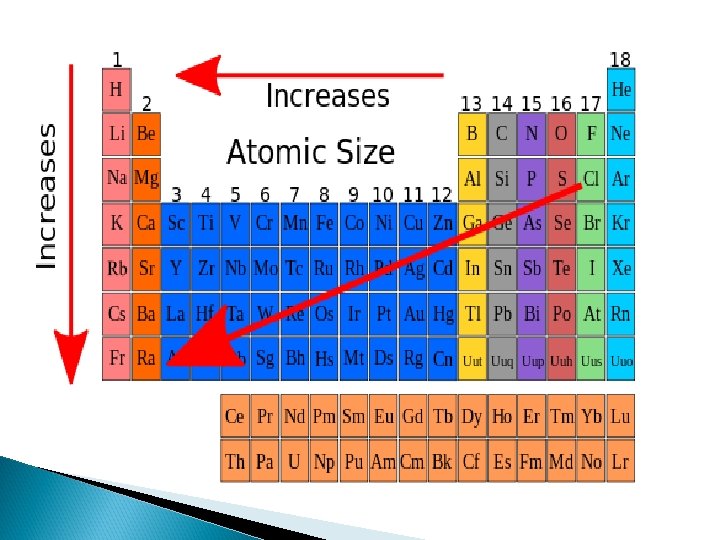
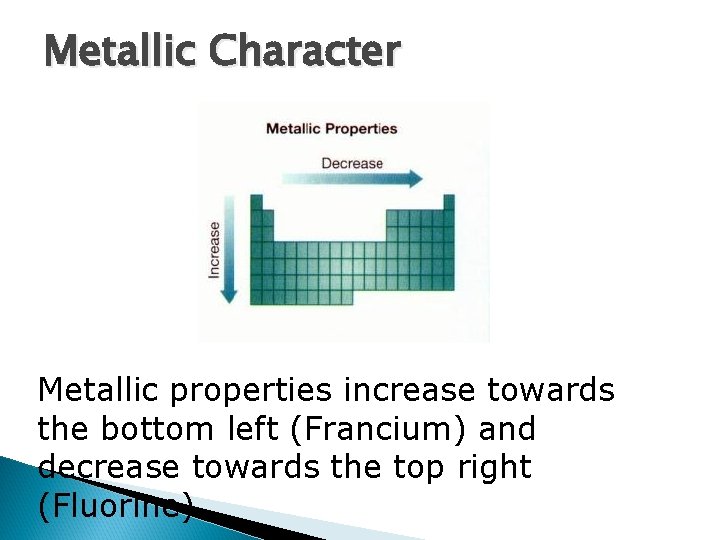
Metallic Character Metallic properties increase towards the bottom left (Francium) and decrease towards the top right (Fluorine).
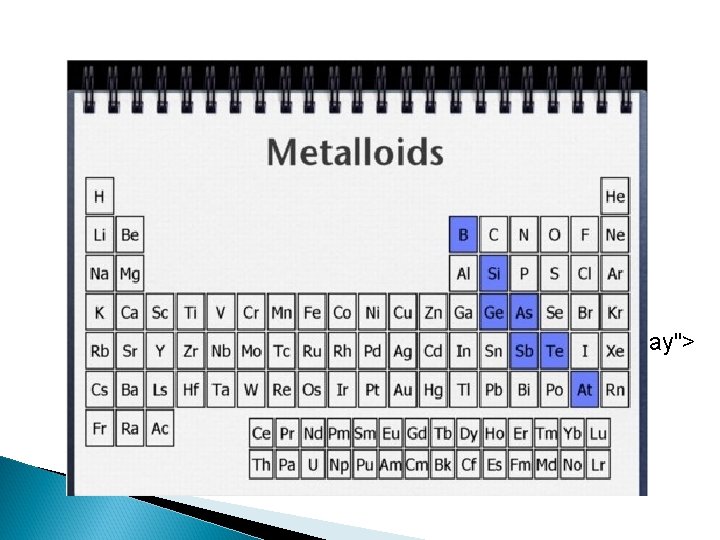
Organizing Information on the Periodic Table Use colored pencils to shade each group or category a different color. n On your handout, draw a stair step dark line starting between B and Al. n Label the right side: nonmetals n Label the left side: metals n Write METALLOID along stair step line (all except for Al and Po!) n
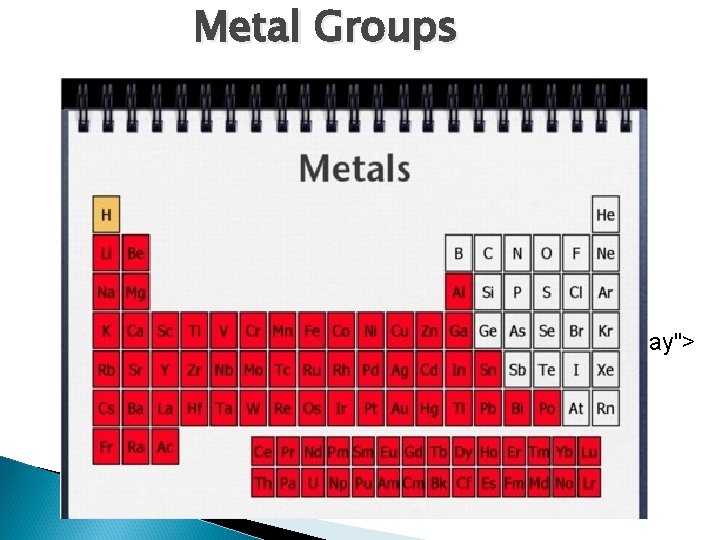
Metal Groups < TARGET="display">
< TARGET="display">
< TARGET="display">

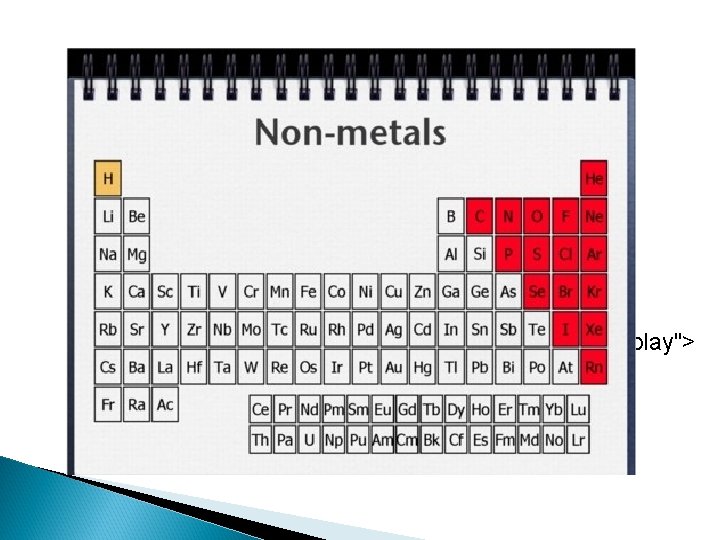
Basic Properties of Metals, Nonmetals, and Metalloids n Metals: 1. malleable, ductile and shiny solids. 2. Conduct heat and electricity well. 3. Tend to give up electrons in reactions (form + ions). n Nonmetals: 1. Generally gases or brittle solids. 2. Solids have dull surface. 3. Good insulators. 4. Tend to gain electrons in reactions (form – ions). n Metalloids: 1. Properties of both metals and nonmetals.
Periodic Table Recall � What are the rows called in a periodic table? Periods � Describe the trend for metallic elements in the periodic table ◦ Increase as you go down table and decrease as you go from left to right across periodic table.

Periodic Table Recall � What element would have a bigger atom Potassium (K) or Neon (N)? Potassium (K)
- Slides: 20