Periodic Table Look for blue circles these will
Periodic Table Look for blue circles: these will tell you how to color periodic table!!
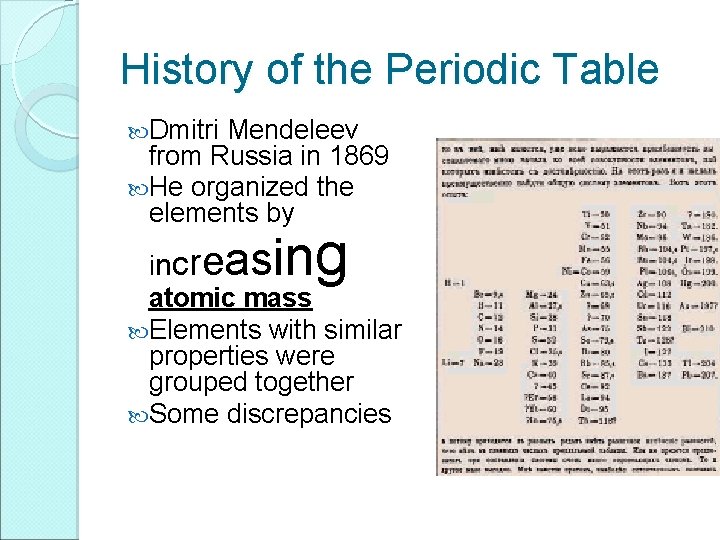
History of the Periodic Table Dmitri Mendeleev from Russia in 1869 He organized the elements by asing incre atomic mass Elements with similar properties were grouped together Some discrepancies

Moseley 40 years after Mendeleev, Moseley arranged the elements by atomic number This lead to the modern periodic table

Mendeleev and Moseley The work of Mendeleev and Mosley led to the discovery of the periodic law Periodic law: there are predictable patterns of physical and chemical properties when elements are arranged in order of increasing atomic number
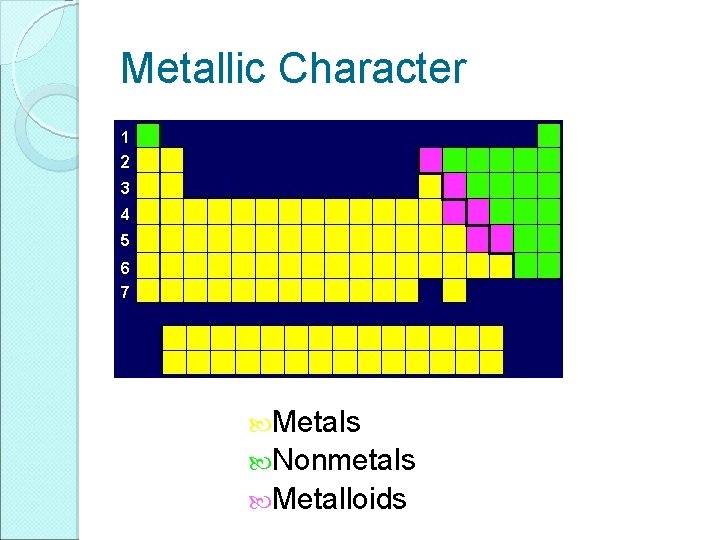
Metallic Character Metals Nonmetals Metalloids

Most Elements are Metals
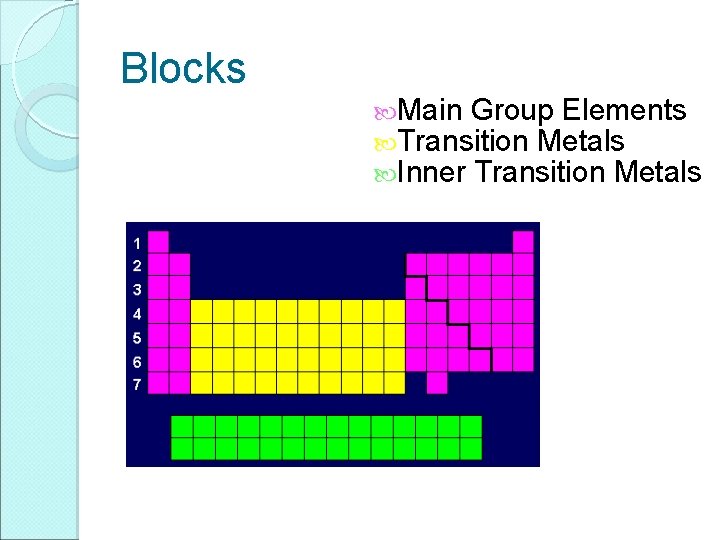
Blocks Main Group Elements Transition Metals Inner Transition Metals
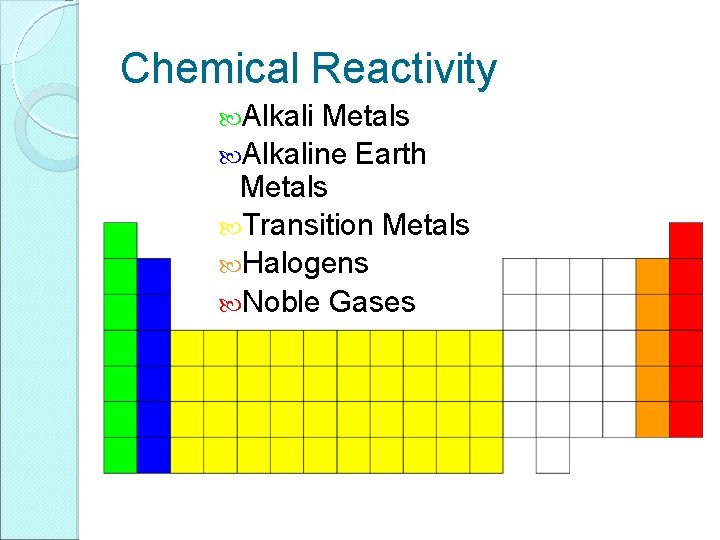
Chemical Reactivity Alkali Metals Alkaline Earth Metals Transition Metals Halogens Noble Gases

Columns The vertical columns of the periodic table (there are 18) are called groups or families Elements in the same group or family have similar properties or characteristics because they have the same number of valence electrons (electrons in outermost energy level)
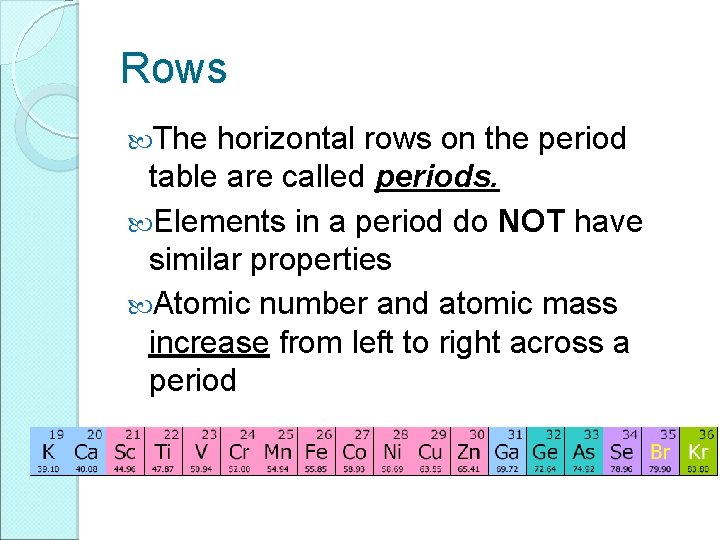
Rows The horizontal rows on the period table are called periods. Elements in a period do NOT have similar properties Atomic number and atomic mass increase from left to right across a period
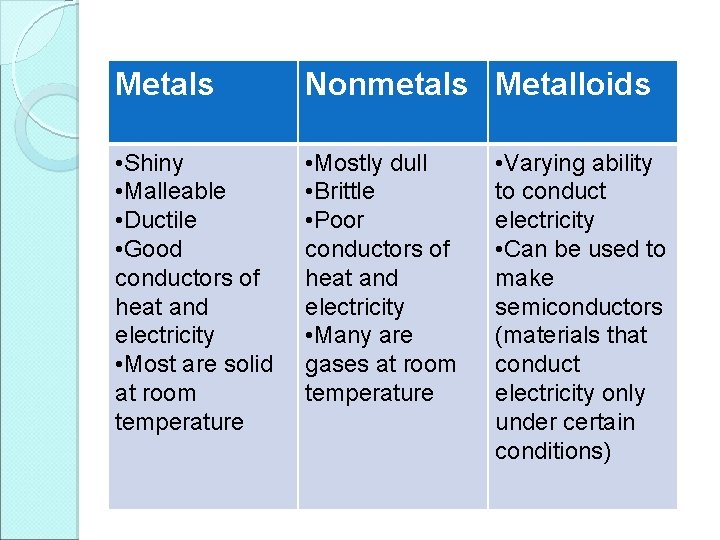
Metals Nonmetals Metalloids • Shiny • Malleable • Ductile • Good conductors of heat and electricity • Most are solid at room temperature • Mostly dull • Brittle • Poor conductors of heat and electricity • Many are gases at room temperature • Varying ability to conduct electricity • Can be used to make semiconductors (materials that conduct electricity only under certain conditions)
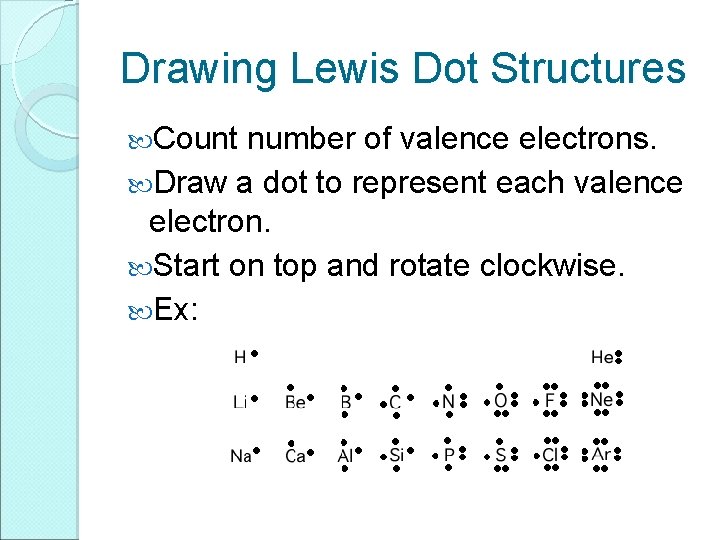
Drawing Lewis Dot Structures Count number of valence electrons. Draw a dot to represent each valence electron. Start on top and rotate clockwise. Ex:

Alkali Metals Located in Group I and have 1 valence electron Physical properties Color these blue!!! ◦ Soft, whitish-silver color ◦ Low-density, conductive, malleable, solid at room temperature Chemical ◦ properties highly reactive, donate one electron when reacting
Alkali Metals
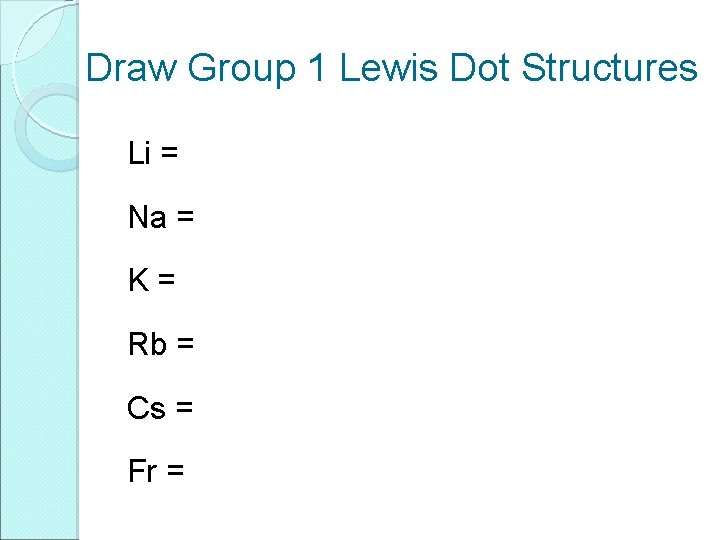
Draw Group 1 Lewis Dot Structures Li = Na = K= Rb = Cs = Fr =
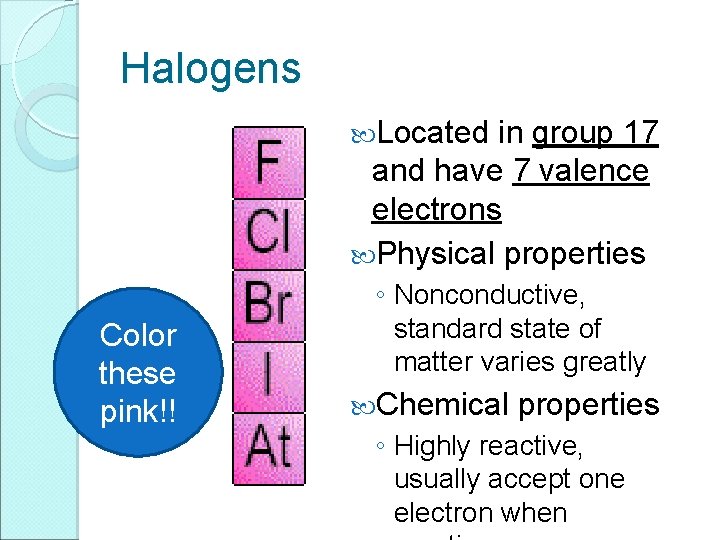
Halogens Located in group 17 and have 7 valence electrons Physical properties Color these pink!! ◦ Nonconductive, standard state of matter varies greatly Chemical properties ◦ Highly reactive, usually accept one electron when

Halogens

Draw Group 17 (Halogens) Lewis Dot Structures F= Cl = Br = I= At =
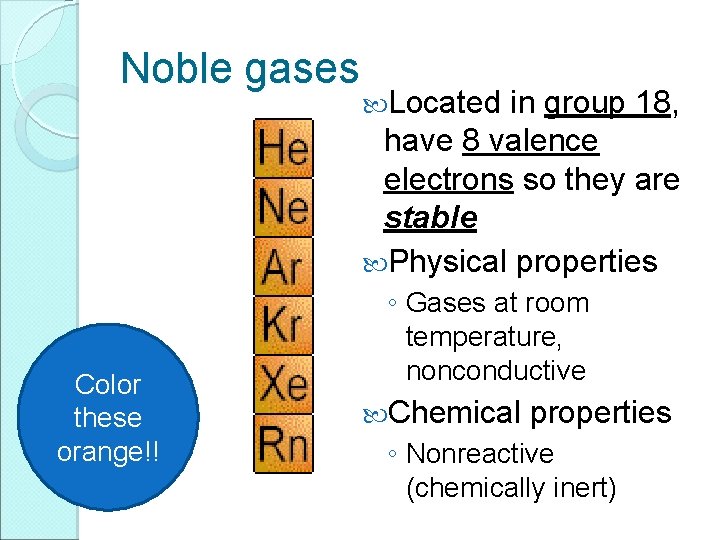
Noble gases Color these orange!! Located in group 18, have 8 valence electrons so they are stable Physical properties ◦ Gases at room temperature, nonconductive Chemical properties ◦ Nonreactive (chemically inert)

Noble Gases
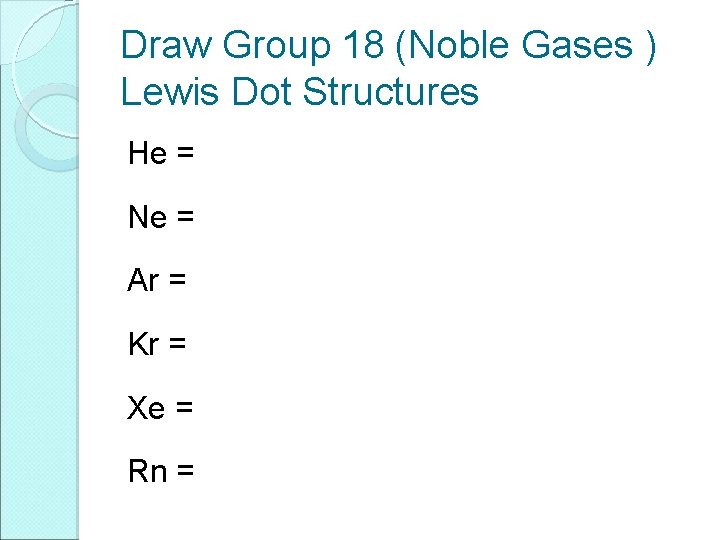
Draw Group 18 (Noble Gases ) Lewis Dot Structures He = Ne = Ar = Kr = Xe = Rn =
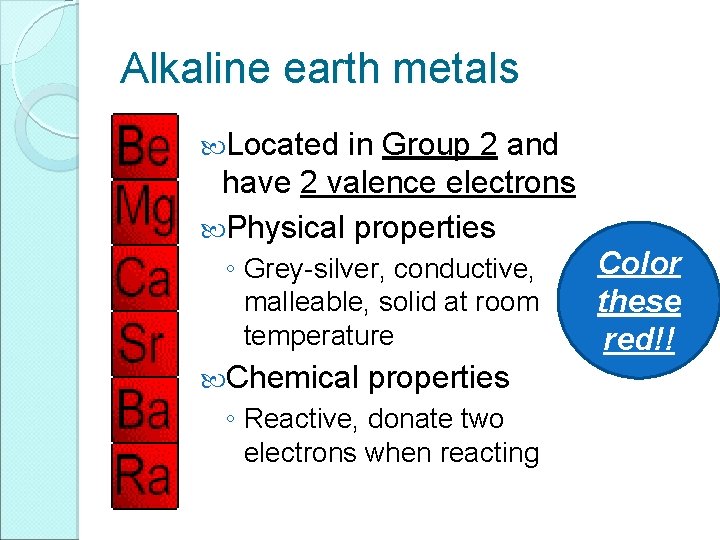
Alkaline earth metals Located in Group 2 and have 2 valence electrons Physical properties ◦ Grey-silver, conductive, malleable, solid at room temperature Chemical properties ◦ Reactive, donate two electrons when reacting Color these red!!

Alkaline earth metals
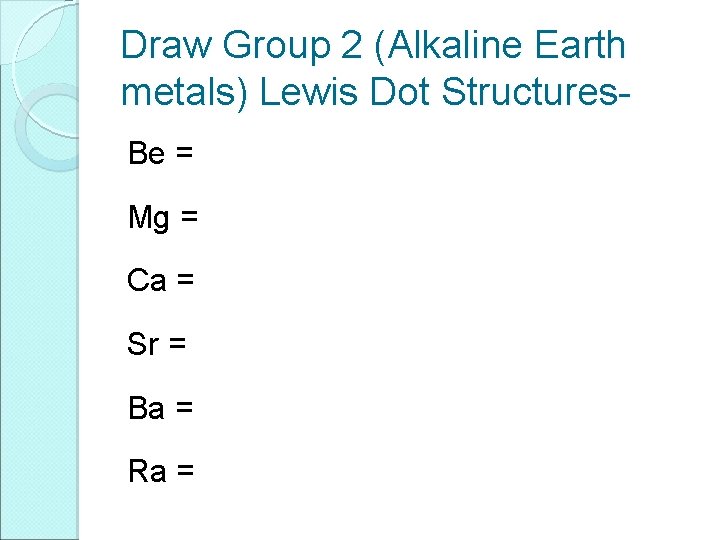
Draw Group 2 (Alkaline Earth metals) Lewis Dot Structures. Be = Mg = Ca = Sr = Ba = Ra =
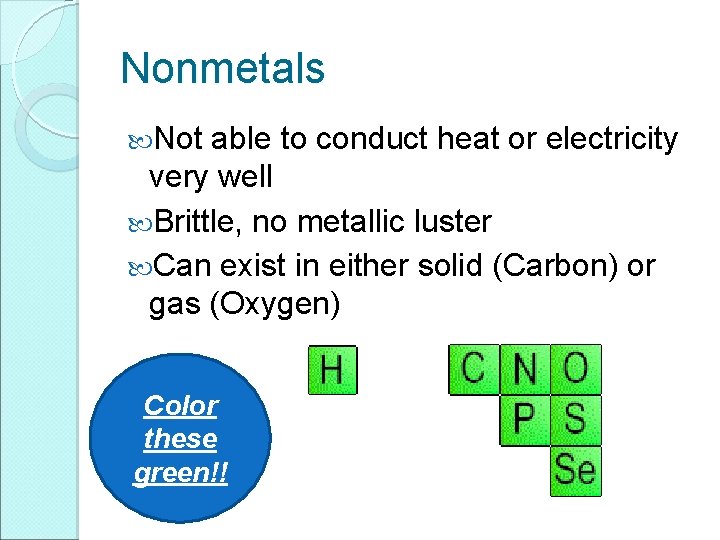
Nonmetals Not able to conduct heat or electricity very well Brittle, no metallic luster Can exist in either solid (Carbon) or gas (Oxygen) Color these green!!

Nonmetals
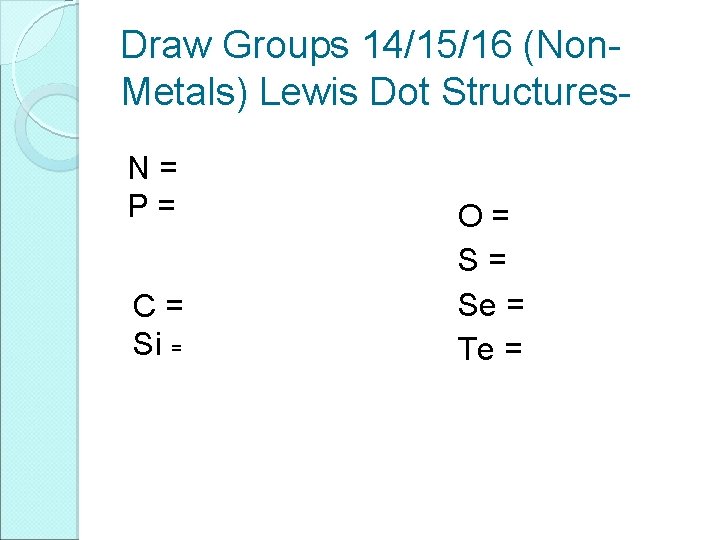
Draw Groups 14/15/16 (Non. Metals) Lewis Dot Structures. N= P= C= Si = O= S= Se = Te =
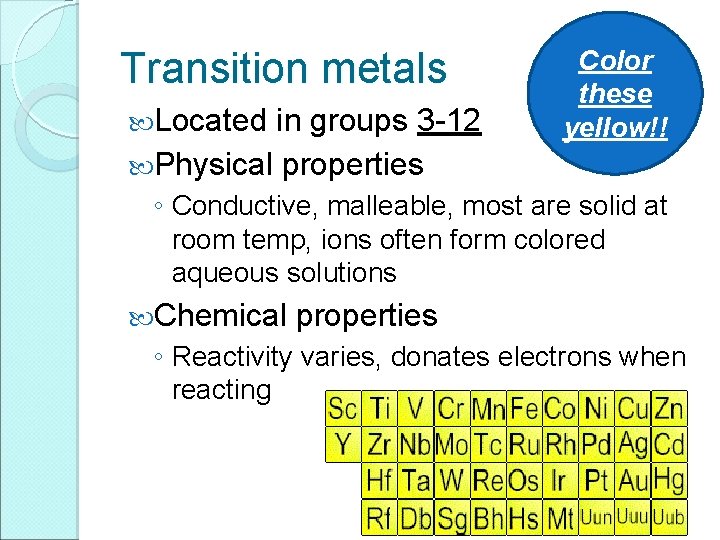
Transition metals Located in groups 3 -12 Physical properties Color these yellow!! ◦ Conductive, malleable, most are solid at room temp, ions often form colored aqueous solutions Chemical properties ◦ Reactivity varies, donates electrons when reacting

Transition Metals
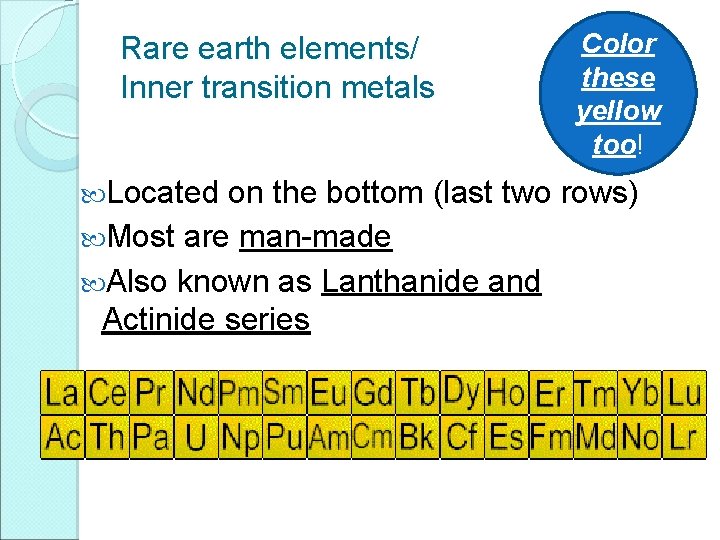
Rare earth elements/ Inner transition metals Located Color these yellow too! on the bottom (last two rows) Most are man-made Also known as Lanthanide and Actinide series

Rare Earth Metals
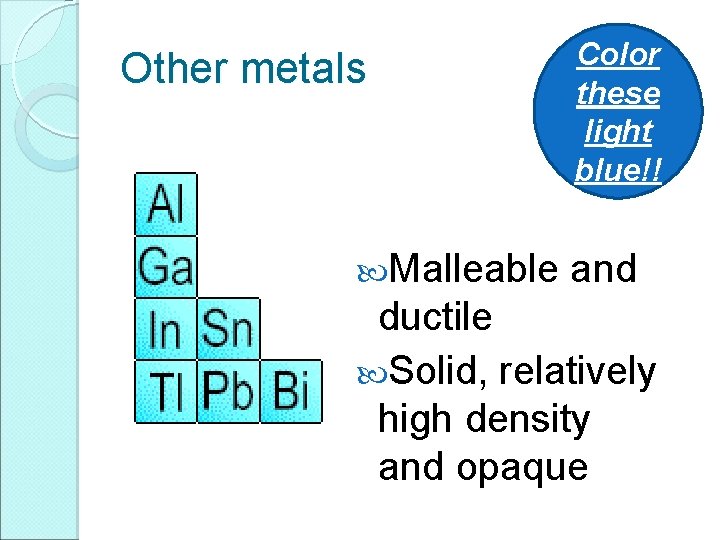
Other metals Malleable Color these light blue!! and ductile Solid, relatively high density and opaque
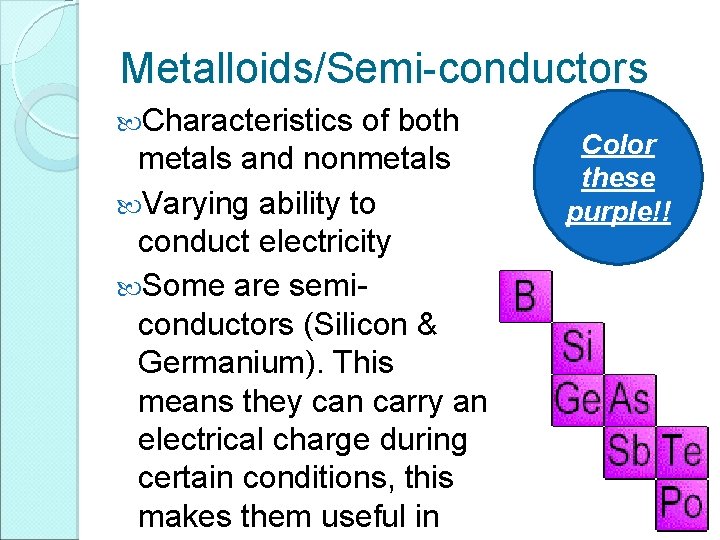
Metalloids/Semi-conductors Characteristics of both metals and nonmetals Varying ability to conduct electricity Some are semiconductors (Silicon & Germanium). This means they can carry an electrical charge during certain conditions, this makes them useful in Color these purple!!

Metalloids
- Slides: 35