Periodic Table Lesson 1 Today you will learn

Periodic Table Lesson 1 – Today you will learn about the periodic table and elements The worksheet on elements and a periodic table are also included as word documents.

Watch the following video about elements in the Periodic Table https: //www. youtube. com/watch? v=rz 4 Dd 1 I _f. X 0

Periodic Table basics. Task 1: Read the information on slides 2 to 5 and then answer the questions on slide 6. v The Periodic Table tells us about elements. v The elements are arranged in order in the Periodic Table. v An element is made of one type of atom only e. g. magnesium v A compound is made of two or more different atoms joined together e. g. water v The horizontal rows are called periods. v The columns going down are called groups. v There is a zigzag line dividing the metals from the non-metals. v Some groups have a collective name for the elements.
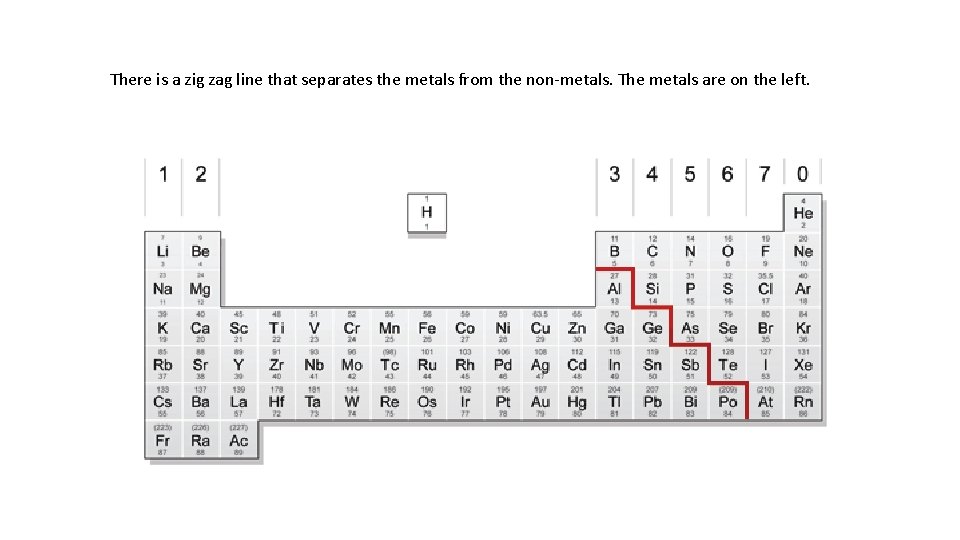
There is a zig zag line that separates the metals from the non-metals. The metals are on the left.
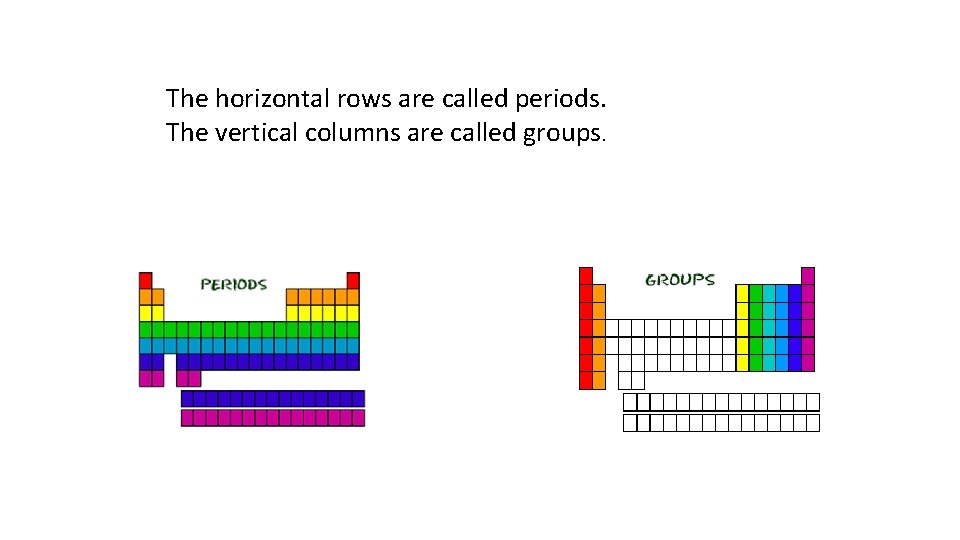
The horizontal rows are called periods. The vertical columns are called groups.
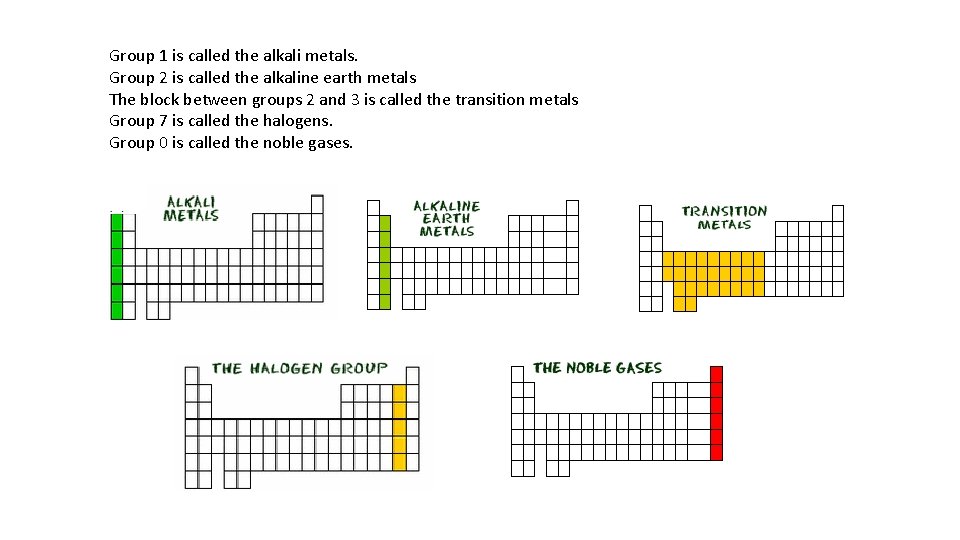
Group 1 is called the alkali metals. Group 2 is called the alkaline earth metals The block between groups 2 and 3 is called the transition metals Group 7 is called the halogens. Group 0 is called the noble gases.
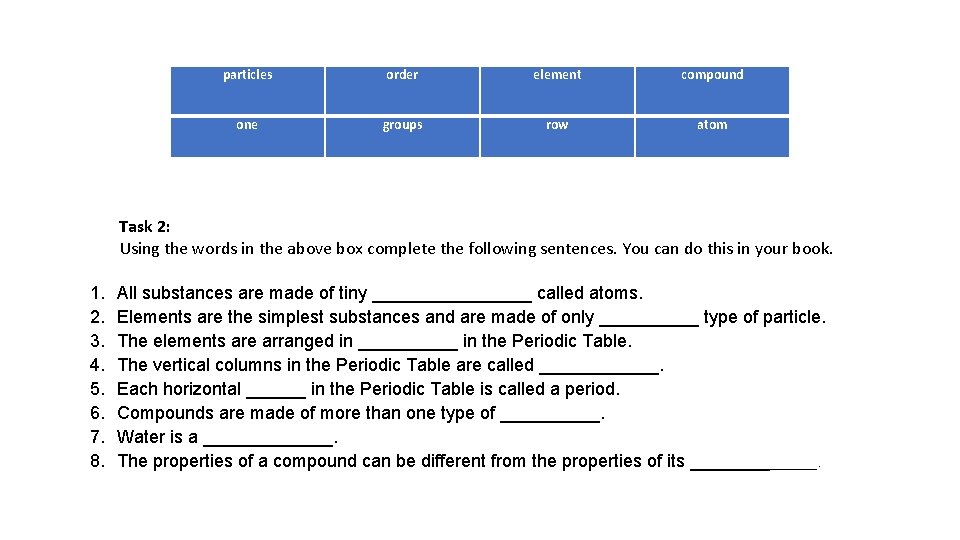
particles order element compound one groups row atom Task 2: Using the words in the above box complete the following sentences. You can do this in your book. 1. 2. 3. 4. 5. 6. 7. 8. All substances are made of tiny ________ called atoms. Elements are the simplest substances and are made of only _____ type of particle. The elements are arranged in _____ in the Periodic Table. The vertical columns in the Periodic Table are called ______. Each horizontal ______ in the Periodic Table is called a period. Compounds are made of more than one type of _____. Water is a _______. The properties of a compound can be different from the properties of its _______.
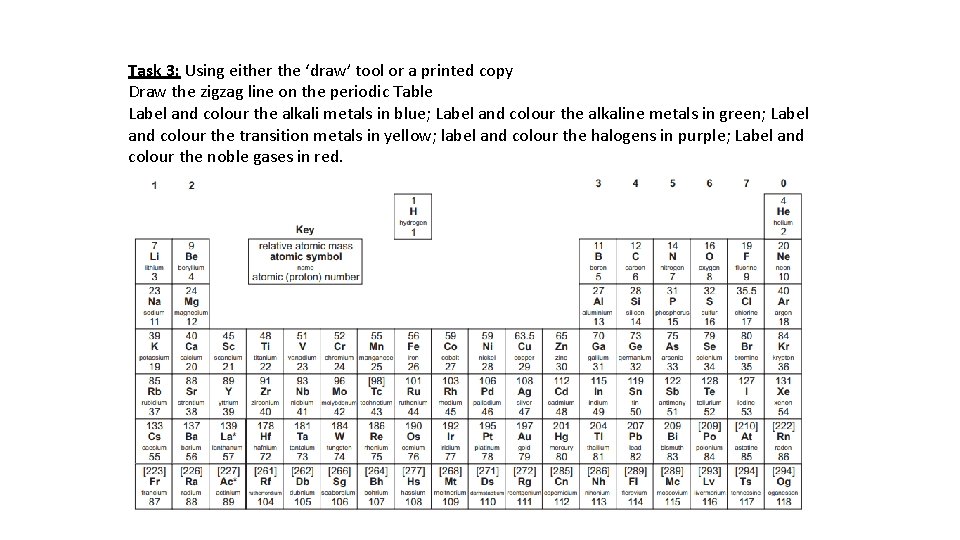
Task 3: Using either the ‘draw’ tool or a printed copy Draw the zigzag line on the periodic Table Label and colour the alkali metals in blue; Label and colour the alkaline metals in green; Label and colour the transition metals in yellow; label and colour the halogens in purple; Label and colour the noble gases in red.
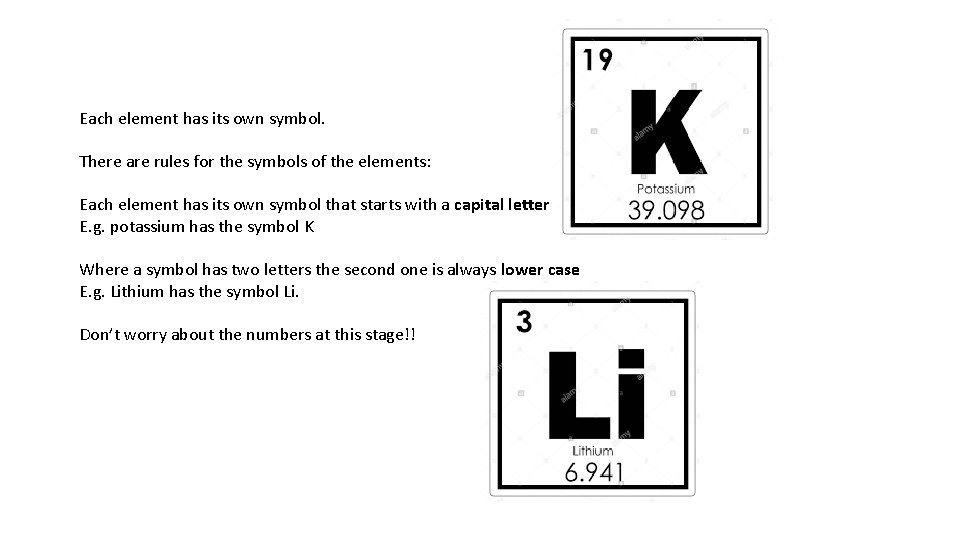
Each element has its own symbol. There are rules for the symbols of the elements: Each element has its own symbol that starts with a capital letter E. g. potassium has the symbol K Where a symbol has two letters the second one is always lower case E. g. Lithium has the symbol Li. Don’t worry about the numbers at this stage!!
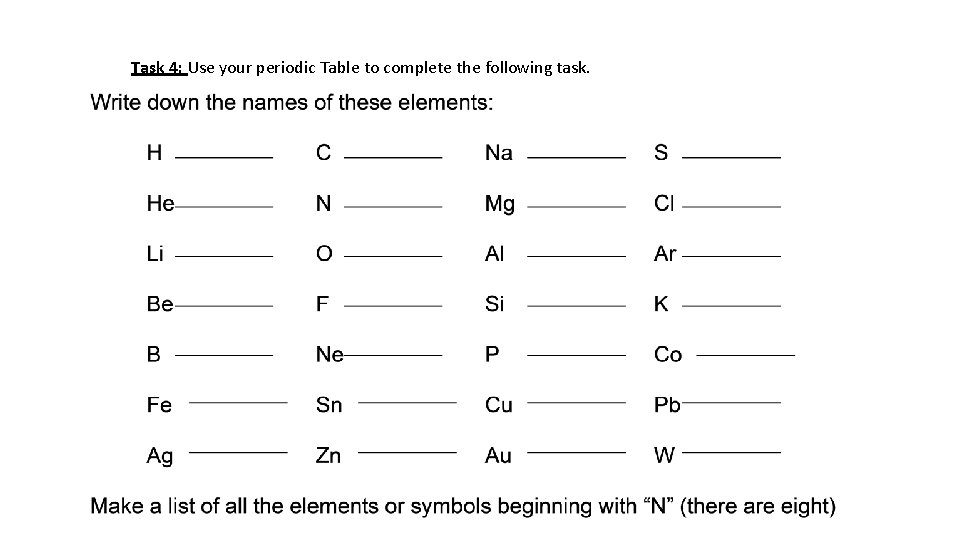
Task 4: Use your periodic Table to complete the following task.

- Slides: 11