Periodic Table II Quantum Numbers All atoms can

Periodic Table II

Quantum Numbers • All atoms can be described by four numbers, called quantum numbers. • Quantum numbers are set by mathematical study of wave characters of electrons. • There are four quantum numbers (n, l, m, ms) that describe an orbital are integers.

Quantum numbers 1. 2. 3. 4. Principal quantum number (n) Orbital quantum number (l) Magnetic quantum number(ml) Spin quantum number (ms )
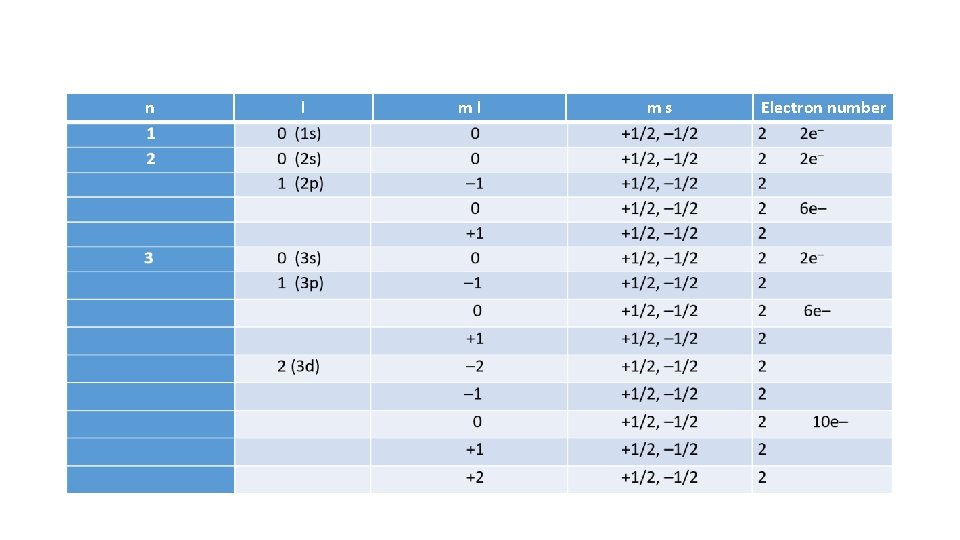
n l ml ms Electron number
![[1 H] = 1 s 1 [2 He] = 1 s 2 [3 Li] [1 H] = 1 s 1 [2 He] = 1 s 2 [3 Li]](http://slidetodoc.com/presentation_image_h2/a525a9a17415db5493e028473b72c50b/image-5.jpg)
[1 H] = 1 s 1 [2 He] = 1 s 2 [3 Li] = 1 s 2 2 s 1 [5 B] = 1 s 2 2 p 1 [6 C] = 1 s 2 2 p 2 [9 F] = 1 s 2 2 p 5 [10 Ne] = 1 s 2 2 p 6 [11 Na] = [Ne] 3 s 1 [12 Mg] = [Ne] 3 s 2 [13 Al] = [Ne] 3 s 2 3 p 1 [14 Si] = [Ne] 3 s 2 3 p 2 [18 Ar] = [Ne] 3 s 2 3 p 6 [19 K] = [Ar] 4 s 1 [20 Ca] = [Ar] 4 s 2
![[21 Sc] = [Ar] 4 s 2 3 d 1 [23 V] = [Ar] [21 Sc] = [Ar] 4 s 2 3 d 1 [23 V] = [Ar]](http://slidetodoc.com/presentation_image_h2/a525a9a17415db5493e028473b72c50b/image-6.jpg)
[21 Sc] = [Ar] 4 s 2 3 d 1 [23 V] = [Ar] 4 s 2 3 d 3 [24 Cr] = [Ar] 4 s 1 3 d 5 [25 Mn] = [Ar] 4 s 2 3 d 5 [26 Fe] = [Ar] 4 s 2 3 d 6 [28 Ni] = [Ar] 4 s 2 3 d 8 [31 Ga] = [Ar] 4 s 2 3 d 10 4 p 1 [32 Ge] = [Ar] 4 s 2 3 d 10 4 p 2 [36 Kr] = [Ar] 4 s 2 3 d 10 4 p 6 [37 Rb] = [Kr] 5 s 1 [38 Sr] = [Kr] 5 s 2
![Actinids [90 Th] = [Rn] 6 d 2 7 s 2 [91 Pa] = Actinids [90 Th] = [Rn] 6 d 2 7 s 2 [91 Pa] =](http://slidetodoc.com/presentation_image_h2/a525a9a17415db5493e028473b72c50b/image-7.jpg)
Actinids [90 Th] = [Rn] 6 d 2 7 s 2 [91 Pa] = [Rn] 5 f 2 6 d 1 7 s 2 [92 U] = [Rn] 5 f 3 6 d 1 7 s 2 [94 Pu] = [Rn] 5 f 6 7 s 2 [95 Am] = [Rn] 5 f 7 7 s 2 [96 Cm] = [Rn] 5 f 7 6 d 1 7 s 2 [97 Bk] = [Rn] 5 f 9 7 s 2 [98 Cf] = [Rn] 5 f 10 7 s 2 [102 No] = [Rn] 5 f 14 7 s 2 [103 Lr] = [Rn] 5 f 14 6 d 1 7 s 2 [104 Rf] = [Rn] 5 f 14 6 d 2 7 s 2
![Noble gases [2 He] [10 Ne] [18 Ar] [36 Kr] [54 Xe] [86 Rn] Noble gases [2 He] [10 Ne] [18 Ar] [36 Kr] [54 Xe] [86 Rn]](http://slidetodoc.com/presentation_image_h2/a525a9a17415db5493e028473b72c50b/image-8.jpg)
Noble gases [2 He] [10 Ne] [18 Ar] [36 Kr] [54 Xe] [86 Rn] = 1 s 2 = [He] 2 s 2 2 p 6 = [Ne] 3 s 2 3 p 6 = [Ar] 3 d 10 4 s 2 4 p 6 = [Kr] 4 d 10 5 s 2 5 p 6 = [Xe] 4 f 14 5 d 10 6 s 2 6 p 6
- Slides: 8