Periodic Table I Ms Hasindu Dassanayake What are
Periodic Table I Ms. Hasindu Dassanayake
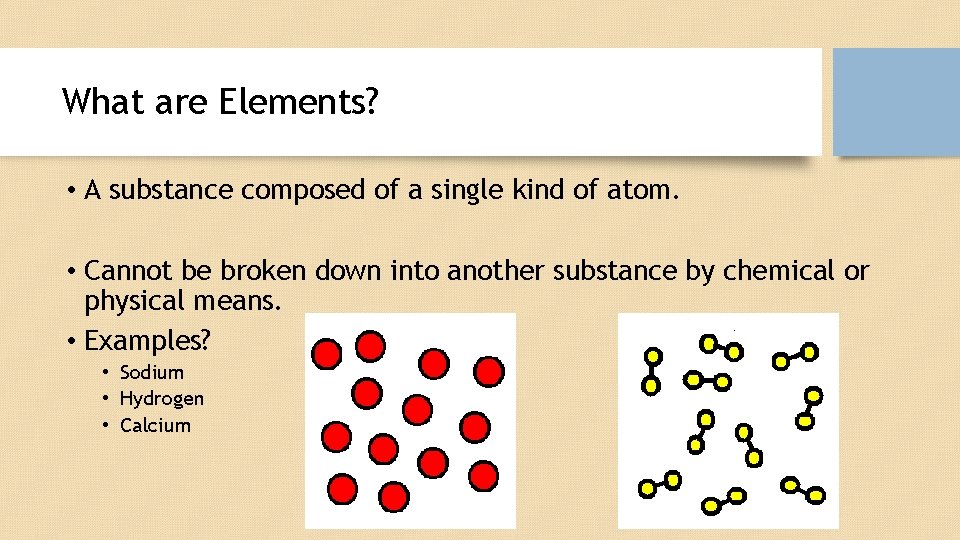
What are Elements? • A substance composed of a single kind of atom. • Cannot be broken down into another substance by chemical or physical means. • Examples? • Sodium • Hydrogen • Calcium
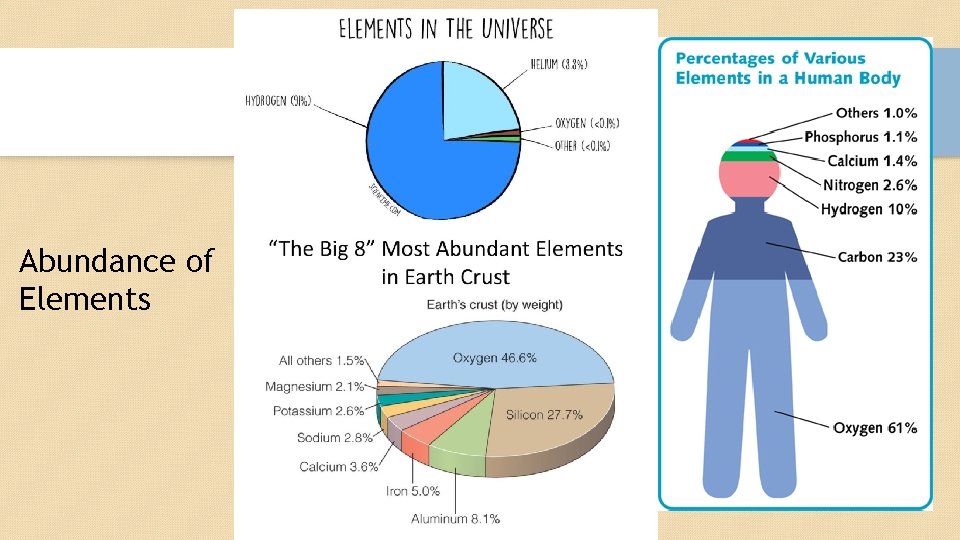
Abundance of Elements
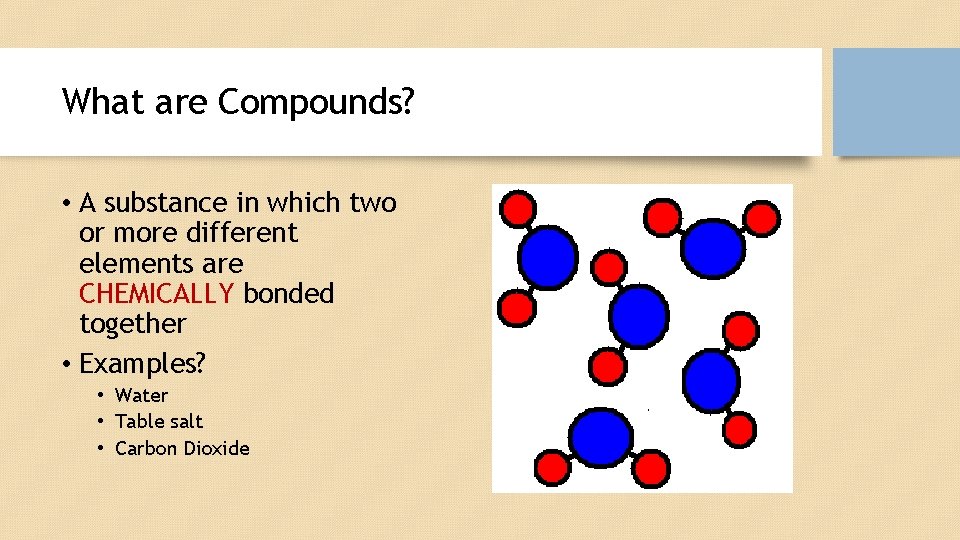
What are Compounds? • A substance in which two or more different elements are CHEMICALLY bonded together • Examples? • Water • Table salt • Carbon Dioxide
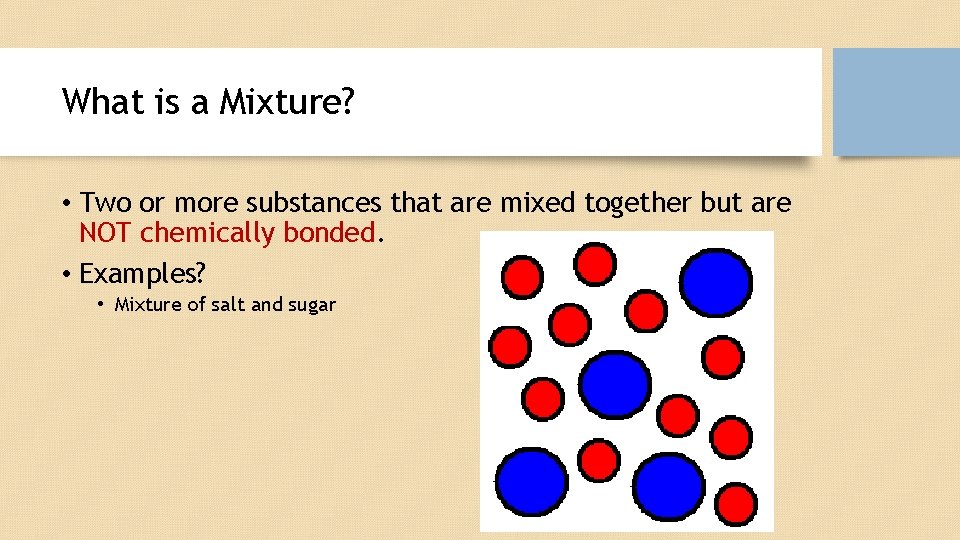
What is a Mixture? • Two or more substances that are mixed together but are NOT chemically bonded. • Examples? • Mixture of salt and sugar
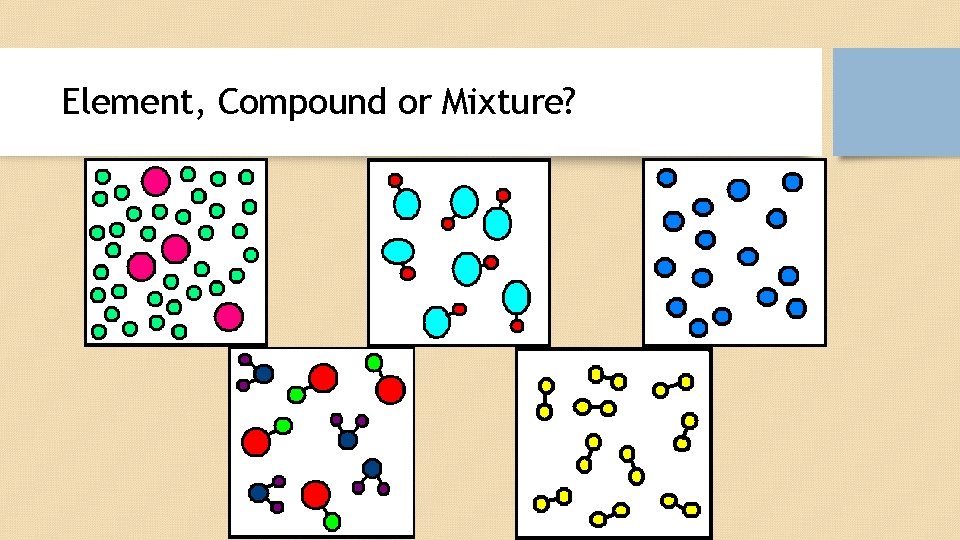
Element, Compound or Mixture?

How do you read the PERIODIC TABLE? • Different periodic tables can include various bits of information, but usually: • atomic number • symbol • atomic mass • number of valence electrons • state of matter at room temperature.

Atomic Number • The atomic number refers to how many protons an atom of that element has. • For instance, hydrogen has 1 proton, so it’s atomic number is 1. • The atomic number is unique to that element. No two elements have the same atomic number. Proton

Atomic Mass • Atomic Mass refers to the “weight” of the atom. • It is derived at by adding the number of protons with the number of neutrons. • This is a helium atom. Its atomic mass is 4 (protons plus neutrons). • What is its atomic number? Protons neutrons and electrons
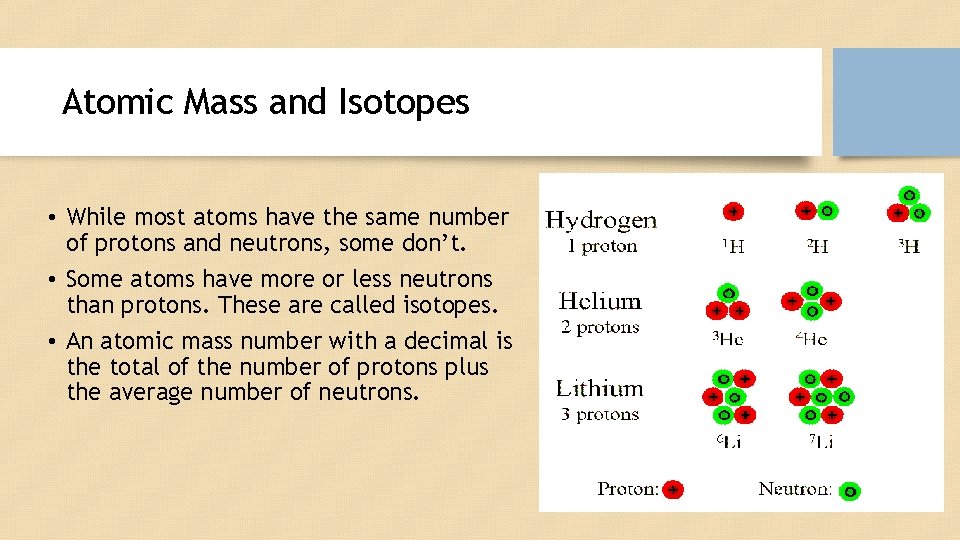
Atomic Mass and Isotopes • While most atoms have the same number of protons and neutrons, some don’t. • Some atoms have more or less neutrons than protons. These are called isotopes. • An atomic mass number with a decimal is the total of the number of protons plus the average number of neutrons.
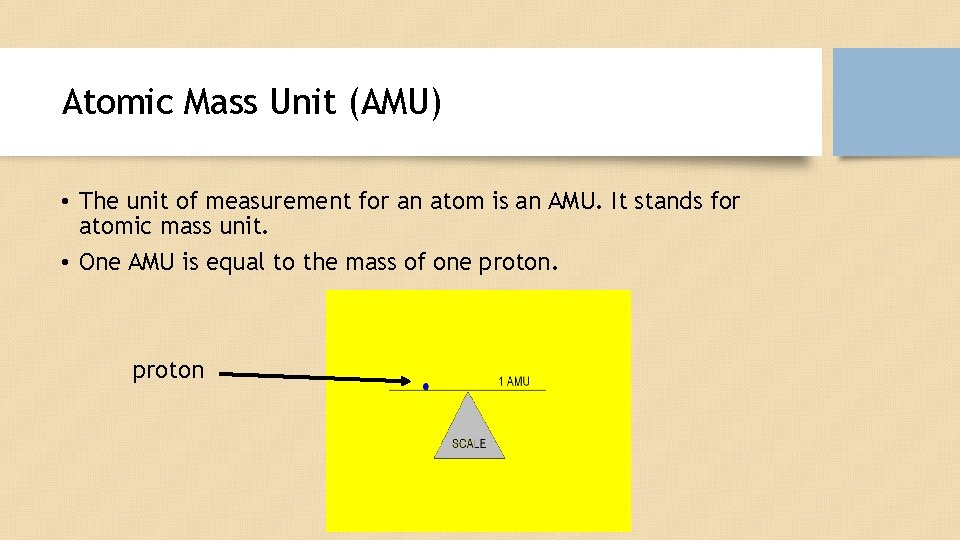
Atomic Mass Unit (AMU) • The unit of measurement for an atom is an AMU. It stands for atomic mass unit. • One AMU is equal to the mass of one proton

Atomic Mass Unit (AMU) • There are 6 X 1023 or 600, 000, 000, 000 amu in one gram. • (Remember that electrons are 2000 times smaller than one amu).
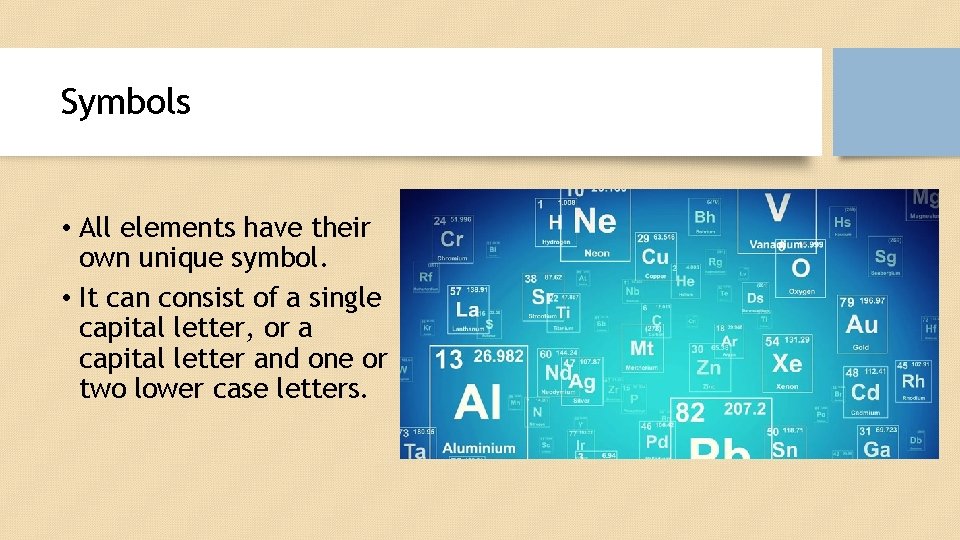
Symbols • All elements have their own unique symbol. • It can consist of a single capital letter, or a capital letter and one or two lower case letters.
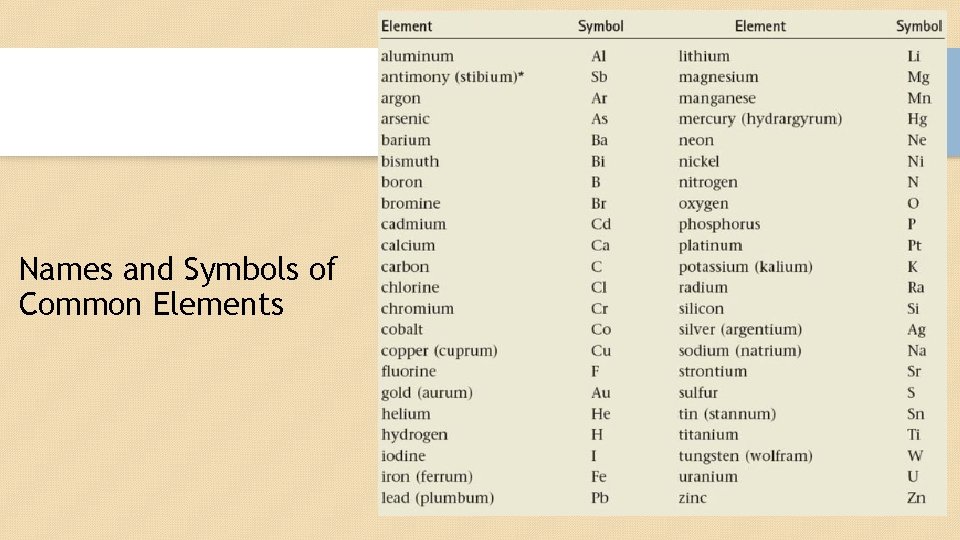
Names and Symbols of Common Elements
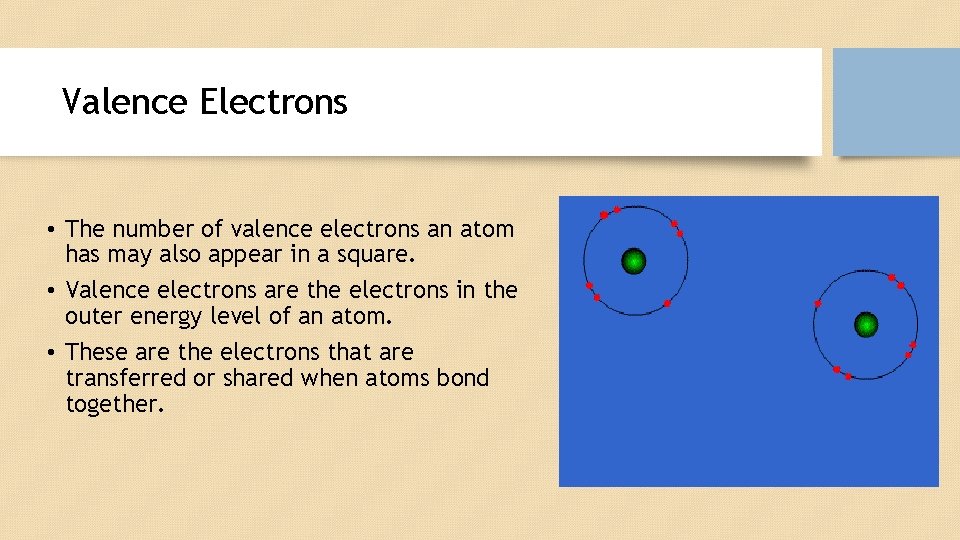
Valence Electrons • The number of valence electrons an atom has may also appear in a square. • Valence electrons are the electrons in the outer energy level of an atom. • These are the electrons that are transferred or shared when atoms bond together.

The Periodic Table • Dmitri Mendelev – 1869 Published a nearly identical classification scheme for elements known to date. • Henry Moseley Said that each element has a unique atomic number. Basis of the current periodic table.
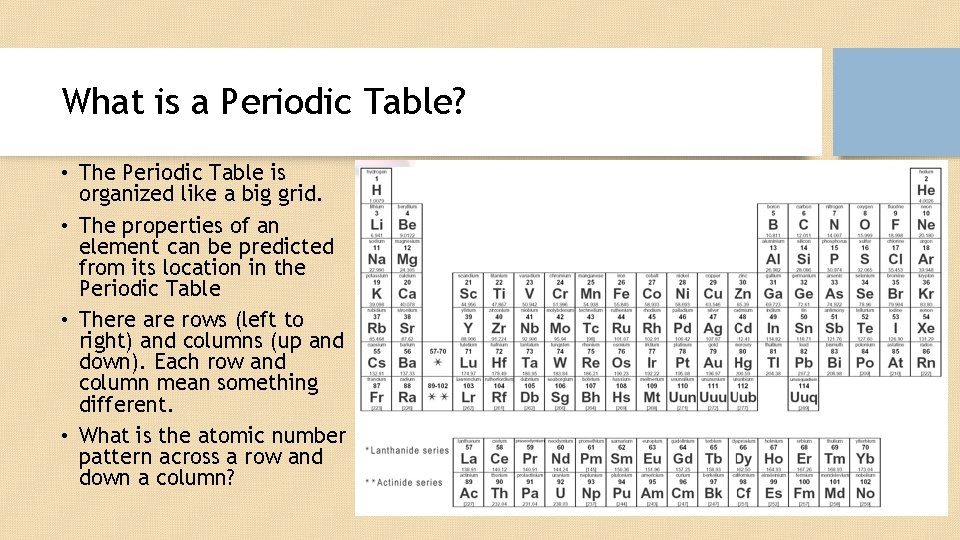
What is a Periodic Table? • The Periodic Table is organized like a big grid. • The properties of an element can be predicted from its location in the Periodic Table • There are rows (left to right) and columns (up and down). Each row and column mean something different. • What is the atomic number pattern across a row and down a column?
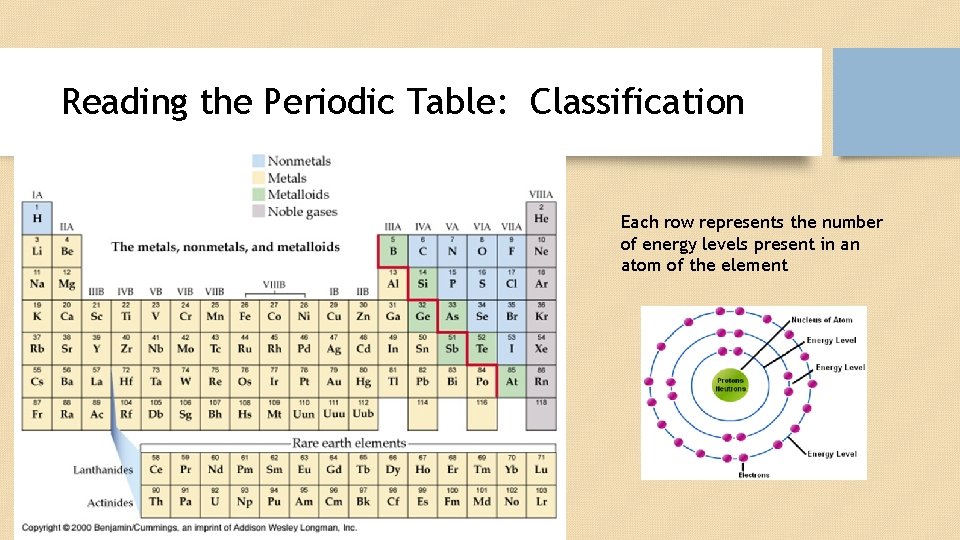
Reading the Periodic Table: Classification Each row represents the number of energy levels present in an atom of the element
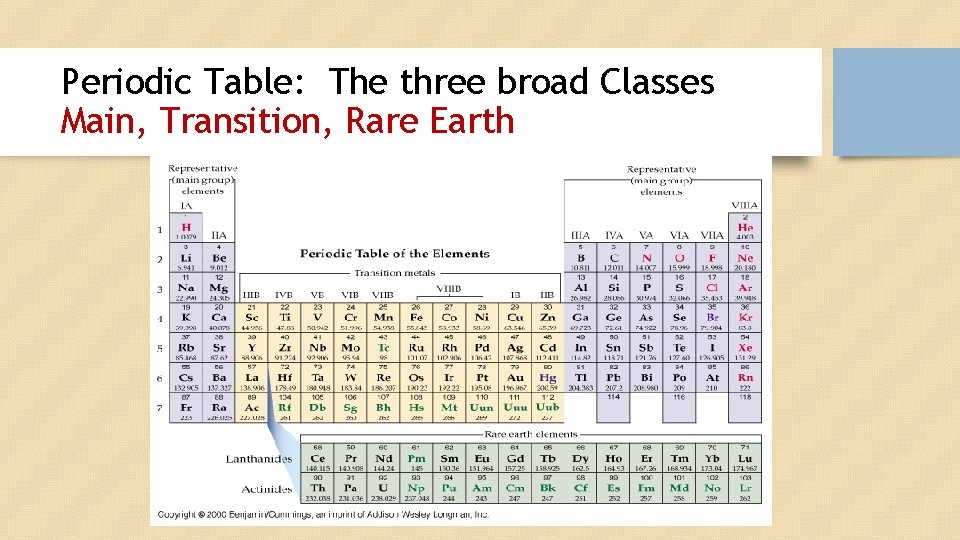
Periodic Table: The three broad Classes Main, Transition, Rare Earth
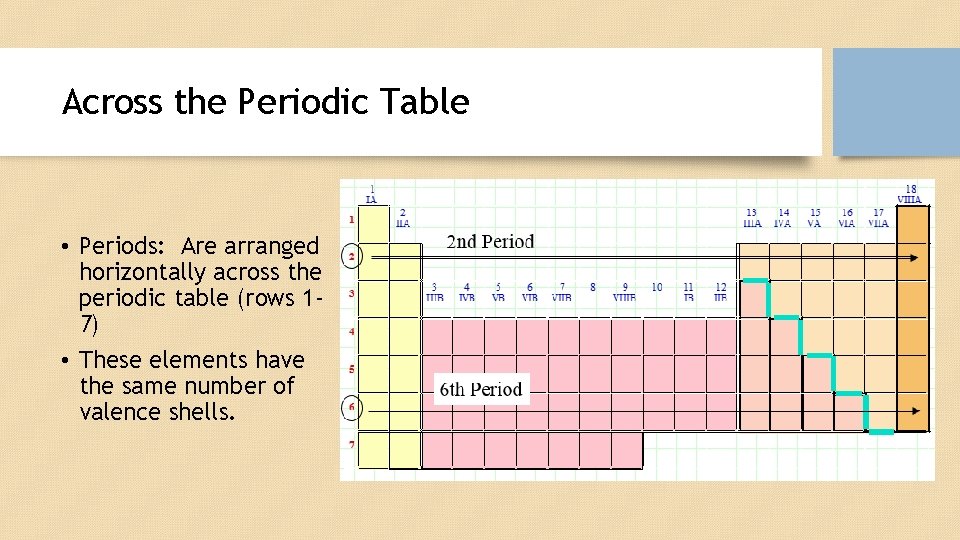
Across the Periodic Table • Periods: Are arranged horizontally across the periodic table (rows 17) • These elements have the same number of valence shells.
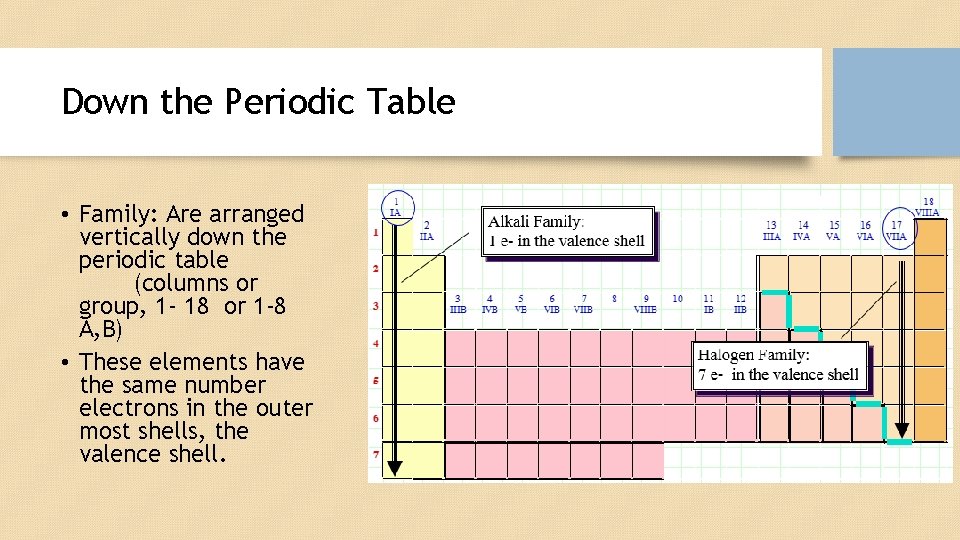
Down the Periodic Table • Family: Are arranged vertically down the periodic table (columns or group, 1 - 18 or 1 -8 A, B) • These elements have the same number electrons in the outer most shells, the valence shell.
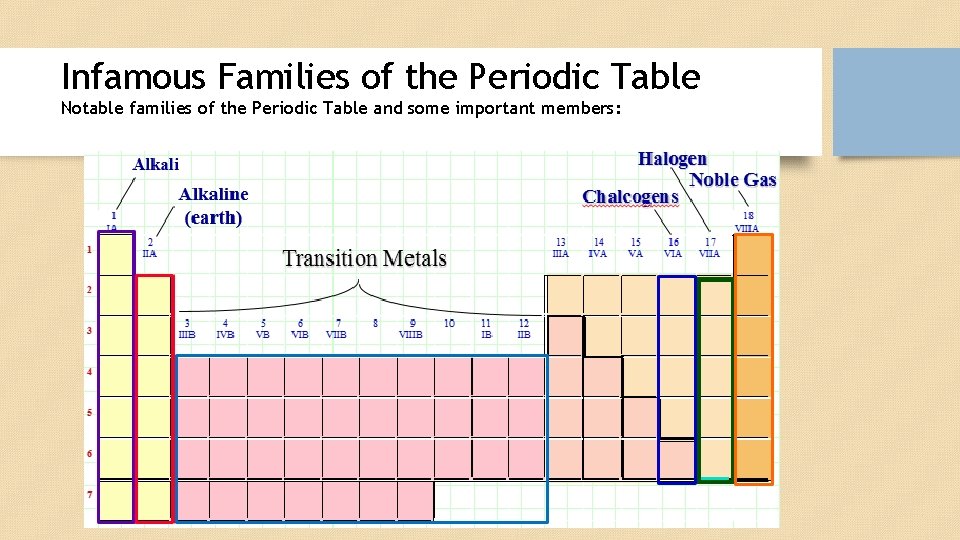
Infamous Families of the Periodic Table Notable families of the Periodic Table and some important members:
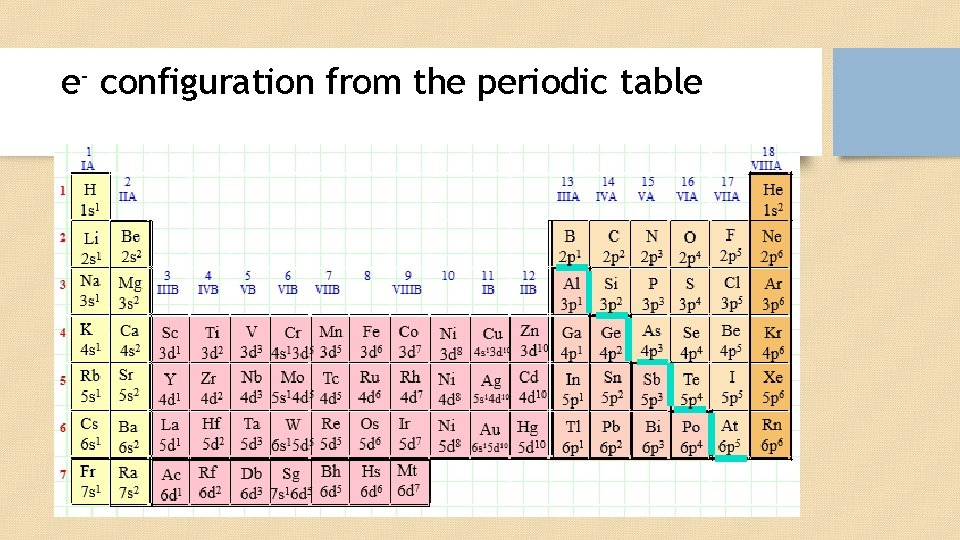
e- configuration from the periodic table
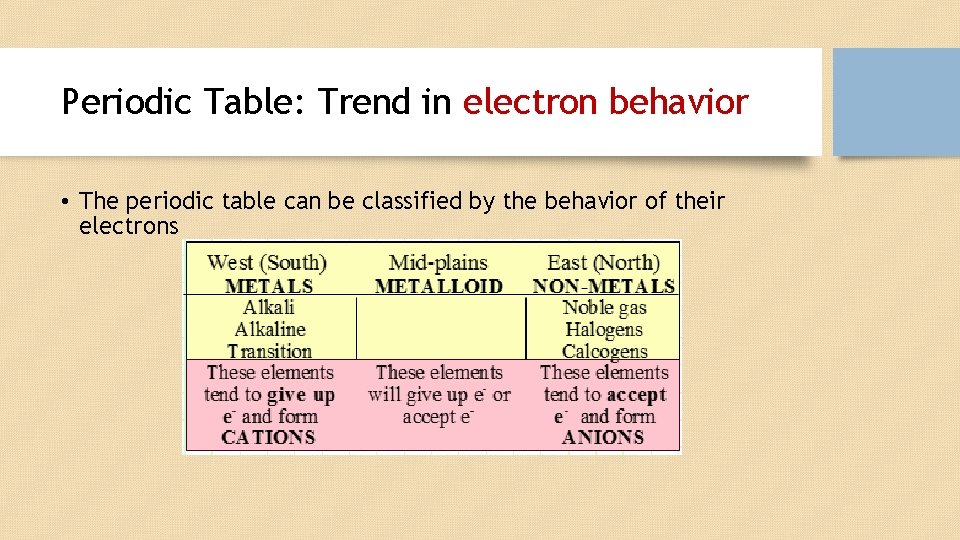
Periodic Table: Trend in electron behavior • The periodic table can be classified by the behavior of their electrons
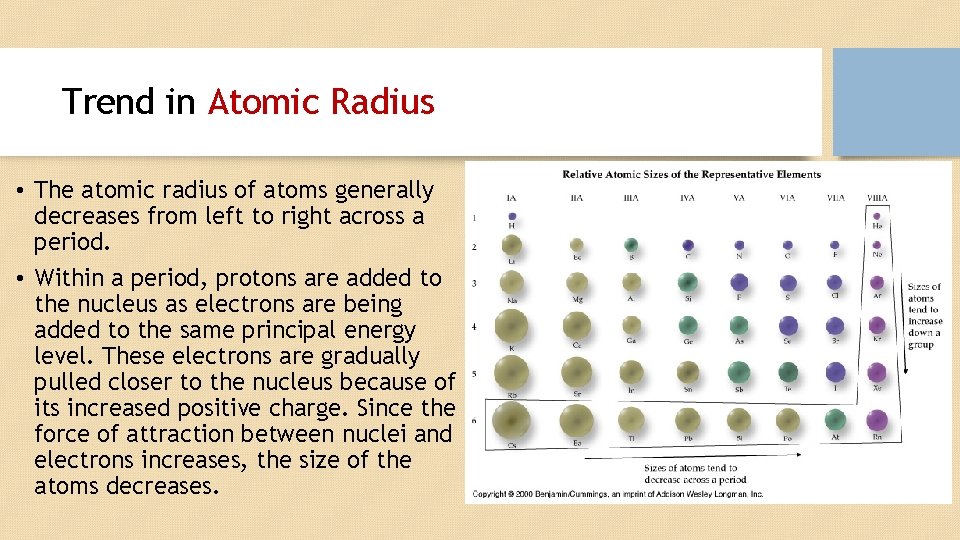
Trend in Atomic Radius • The atomic radius of atoms generally decreases from left to right across a period. • Within a period, protons are added to the nucleus as electrons are being added to the same principal energy level. These electrons are gradually pulled closer to the nucleus because of its increased positive charge. Since the force of attraction between nuclei and electrons increases, the size of the atoms decreases.

Trend in Ionization Potential • Ionization energy is the energy required to remove an electron from a gaseous atom or ion. • Ionization energy generally increases moving from left to right across an element period (row). This is because the atomic radius generally decreases moving across a period, so there is a greater effective attraction between the negatively charged electrons and positively-charged nucleus. • Ionization decreases moving top to bottom down an element group (column).
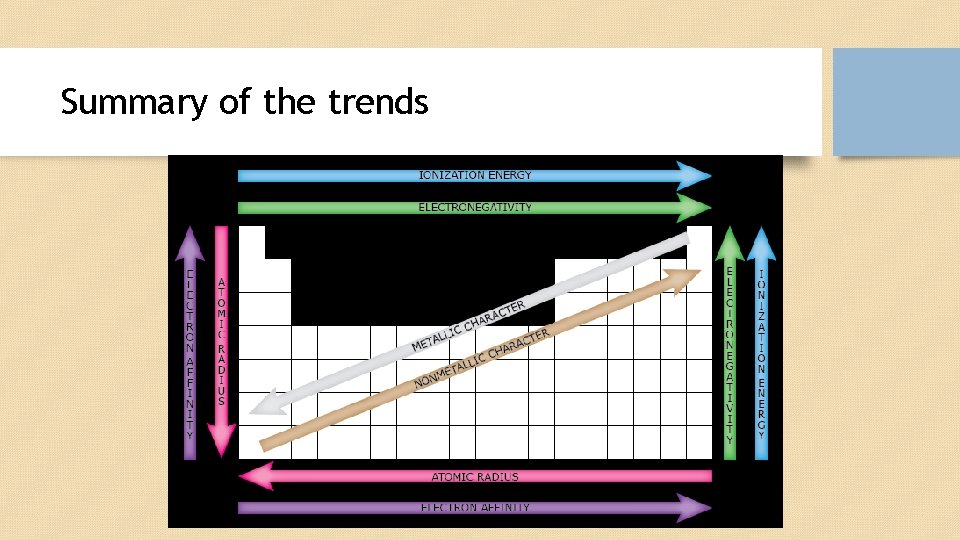
Summary of the trends

Summary • Periodic Table: Map of the Building block of matter • Type: Metal, metalloid and Nonmetal Representative or main, transition and Lanthanide/Actanides • Family: Elements in the same column have similar chemical property because of similar valence electrons Alkali, Alkaline, chalcogens, halogens, noble gases • Period: Elements in the same row have valence electrons in the same shell.
- Slides: 28