Periodic Table How does the periodic table organize

Periodic Table How does the periodic table organize the elements?

History of Atom Science classroom https: //www. youtube. com/watch? v=IO 9 WS_HN myg Extra video on history of atom https: //www. youtube. com/watch? v=NSAg. Lv. KO PLQ
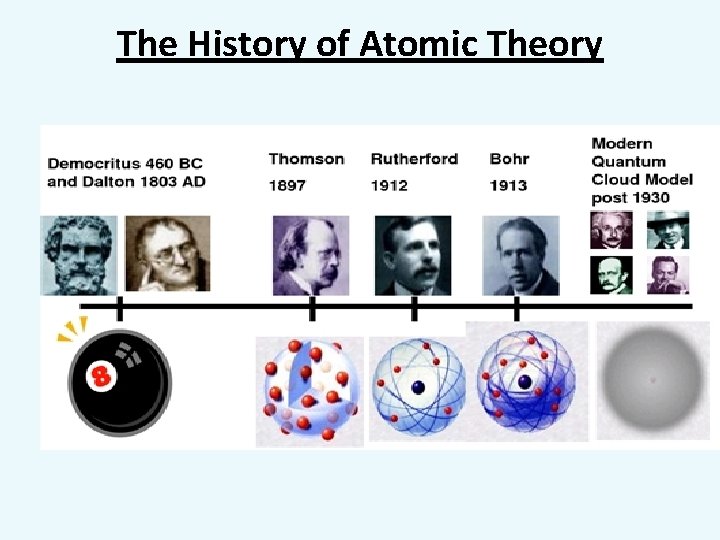
The History of Atomic Theory
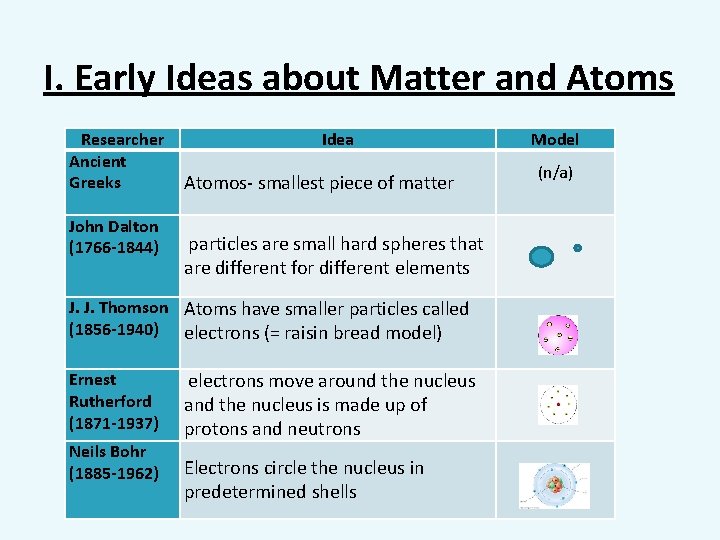
I. Early Ideas about Matter and Atoms Researcher Ancient Greeks John Dalton (1766 -1844) Idea Atomos- smallest piece of matter particles are small hard spheres that are different for different elements J. J. Thomson Atoms have smaller particles called (1856 -1940) electrons (= raisin bread model) Ernest Rutherford (1871 -1937) Neils Bohr (1885 -1962) electrons move around the nucleus and the nucleus is made up of protons and neutrons Electrons circle the nucleus in predetermined shells Model (n/a)

Ancient Greeks • Greek philosopher Democritus believed everything was made up of tiny particles in empty space. • He called them Atomos, meaning “Uncuttable”. • He did not use experiments to support his ideas, just reason and logic. • Philosopher Aristotle disagreed because he didn’t believe empty space could exist. • Denial of existence of atoms persisted for 2000 yr

Dalton’s Atomic Theory 1. All matter is made of small particles called atoms. 2. Atoms cannot be created, destroyed, or divided into smaller particles. 3. All atoms of the same element are identical in mass and size, but they are different in mass and size from the atoms of other elements. 4. Compounds are created when atoms of different elements link together in definite proportions.
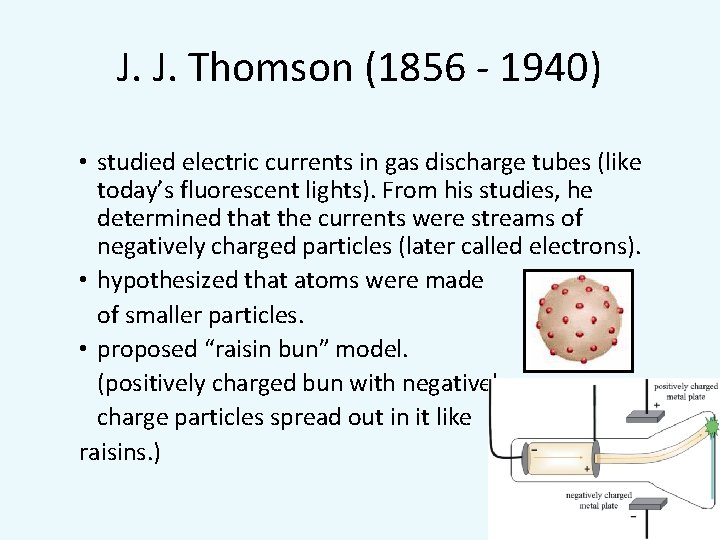
J. J. Thomson (1856 - 1940) • studied electric currents in gas discharge tubes (like today’s fluorescent lights). From his studies, he determined that the currents were streams of negatively charged particles (later called electrons). • hypothesized that atoms were made of smaller particles. • proposed “raisin bun” model. (positively charged bun with negatively charge particles spread out in it like raisins. )
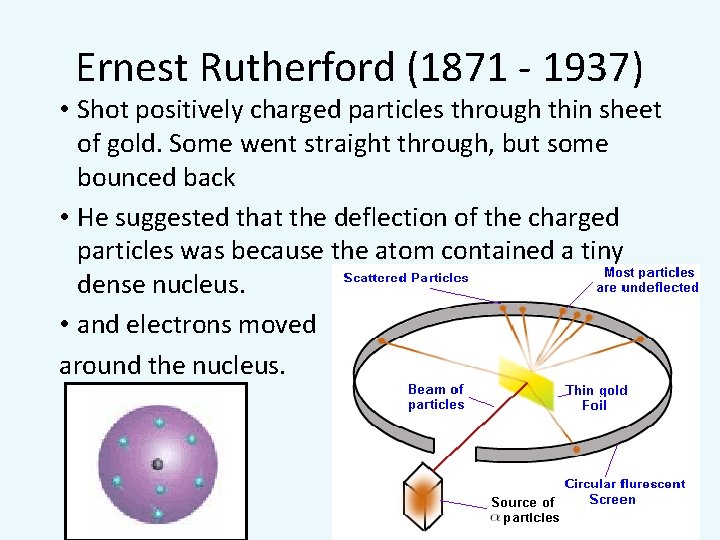
Ernest Rutherford (1871 - 1937) • Shot positively charged particles through thin sheet of gold. Some went straight through, but some bounced back • He suggested that the deflection of the charged particles was because the atom contained a tiny dense nucleus. • and electrons moved around the nucleus.
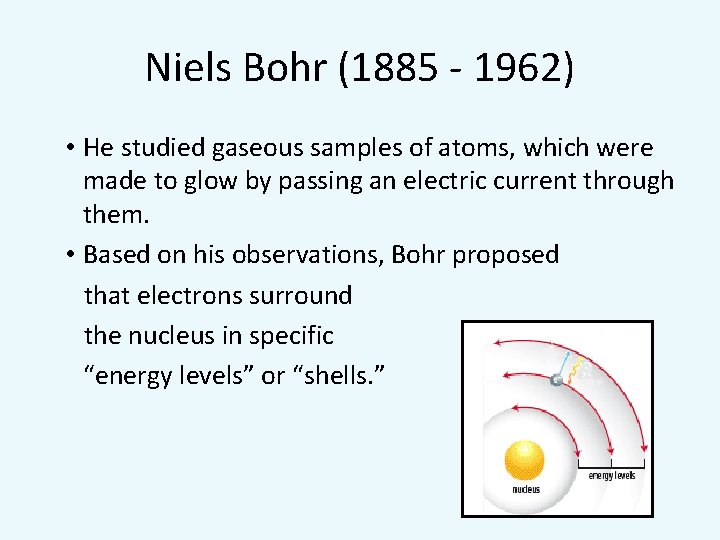
Niels Bohr (1885 - 1962) • He studied gaseous samples of atoms, which were made to glow by passing an electric current through them. • Based on his observations, Bohr proposed that electrons surround the nucleus in specific “energy levels” or “shells. ”

What is an atom? • the basic unit of a chemical element.
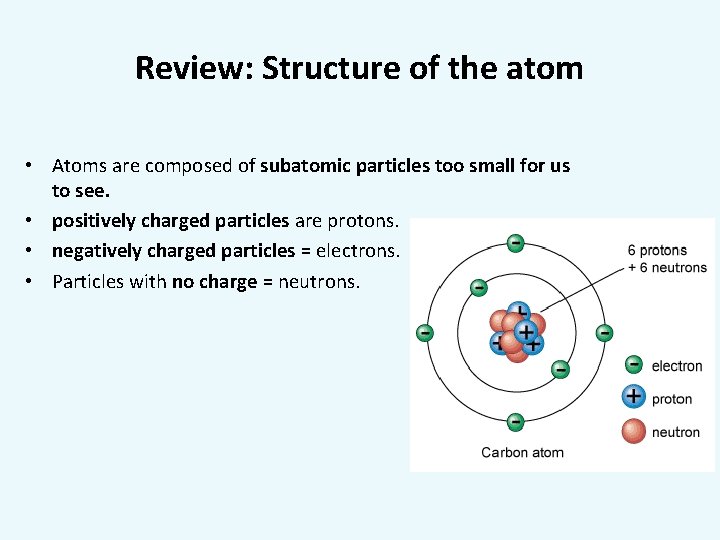
Review: Structure of the atom • Atoms are composed of subatomic particles too small for us to see. • positively charged particles are protons. • negatively charged particles = electrons. • Particles with no charge = neutrons.
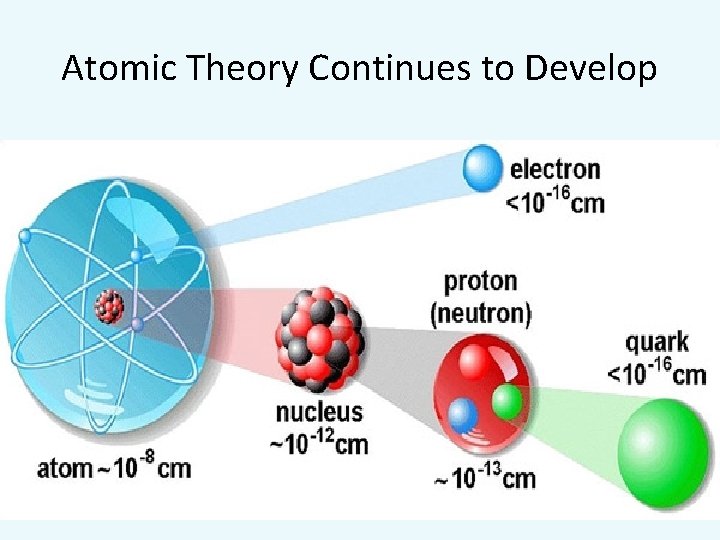
Atomic Theory Continues to Develop

The Atom What is an atom? https: //www. youtube. com/watch? v=o-3 I 1 JGWCk Ted Ed: How small is an atom? https: //www. youtube. com/watch? v=y. QP 4 UJh. N n 0 I

Some have a charge

Check your Understanding 1. What are three subatomic particles? 2. Compare and contrast the electron and proton

Homework • Read p 14 -15, complete atomic history chart in notes. • Answer pages 16 -18 of Work Book handout.
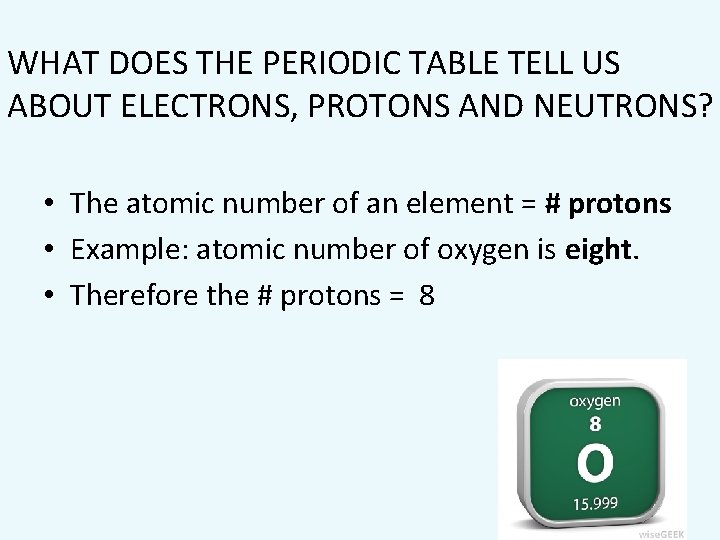
WHAT DOES THE PERIODIC TABLE TELL US ABOUT ELECTRONS, PROTONS AND NEUTRONS? • The atomic number of an element = # protons • Example: atomic number of oxygen is eight. • Therefore the # protons = 8

Element • An element is a pure substance that cannot be broken down or separated into anything simpler than it already is. • Eg. Gold, carbon
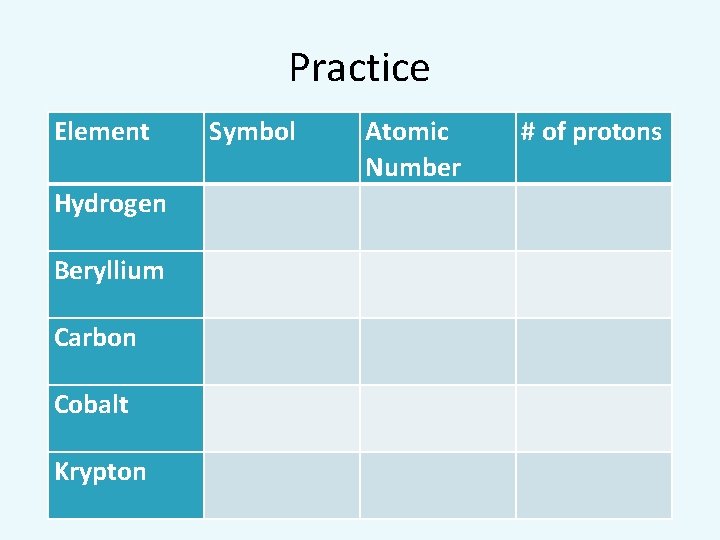
Practice Element Hydrogen Beryllium Carbon Cobalt Krypton Symbol Atomic Number # of protons
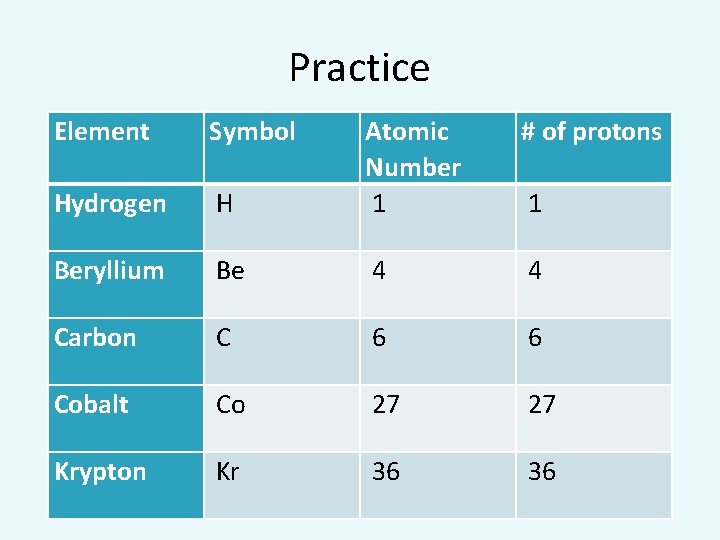
Practice Element Symbol # of protons H Atomic Number 1 Hydrogen Beryllium Be 4 4 Carbon C 6 6 Cobalt Co 27 27 Krypton Kr 36 36 1
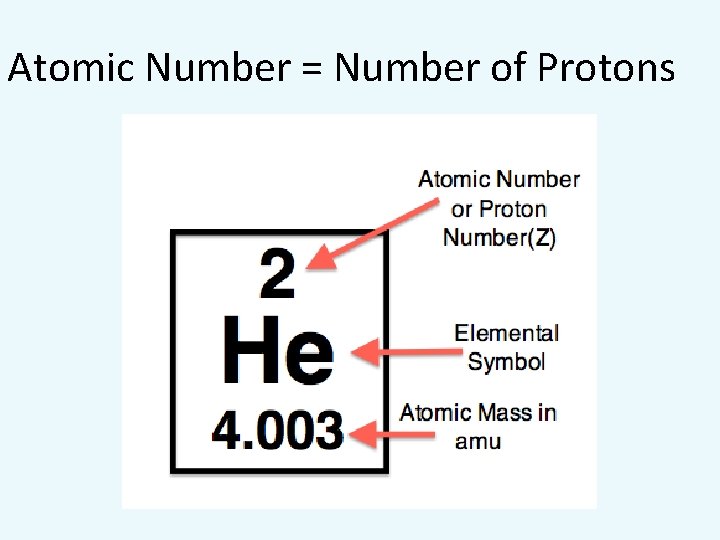
Atomic Number = Number of Protons
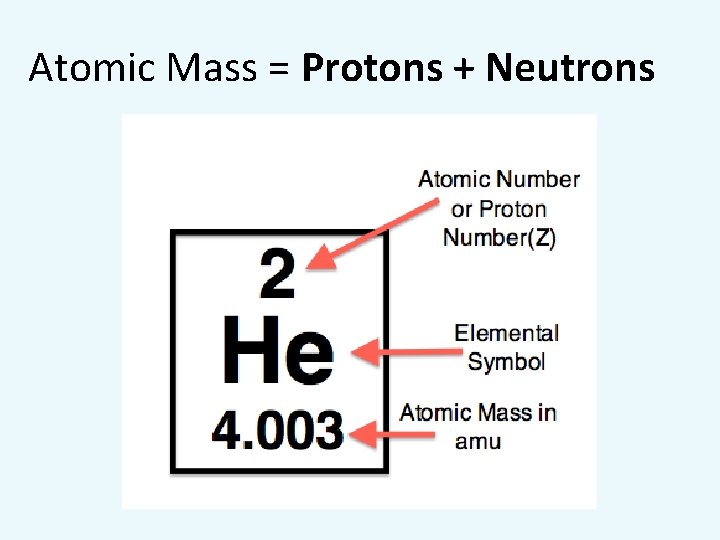
Atomic Mass = Protons + Neutrons
Practice Element Hydrogen Chromium Carbon Barium iron Symbol Mass # # Protons # Neutrons
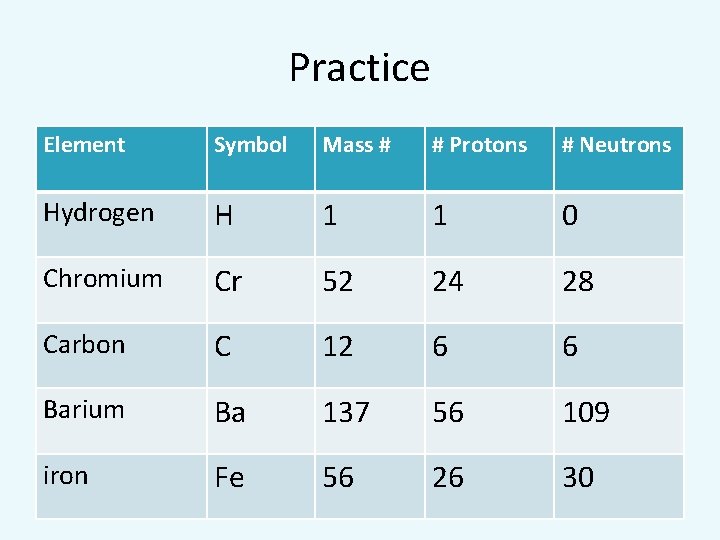
Practice Element Symbol Mass # # Protons # Neutrons Hydrogen H 1 1 0 Chromium Cr 52 24 28 Carbon C 12 6 6 Barium Ba 137 56 109 iron Fe 56 26 30
ELEMENT Lithium Boron Oxygen Phosphorous Argon Nickel Bromine Cobalt Platinum Lead Barium Iron Nitrogen Neon Sulphur Magnesium Potassium ATOMIC # MASS # PROTONS # ELECTRONS # NEUTRONS
ELEMENT Lithium Boron Oxygen Phosphorous Argon Nickel Bromine Cobalt Platinum Lead Barium Iron Nitrogen Neon Sulphur Magnesium Potassium ATOMIC # MASS # PROTONS # ELECTRONS # NEUTRONS
What kinds of elements are there? • The Periodic Table groups elements into families (vertical groups). • A chemical family is a group of elements that share similar properties.
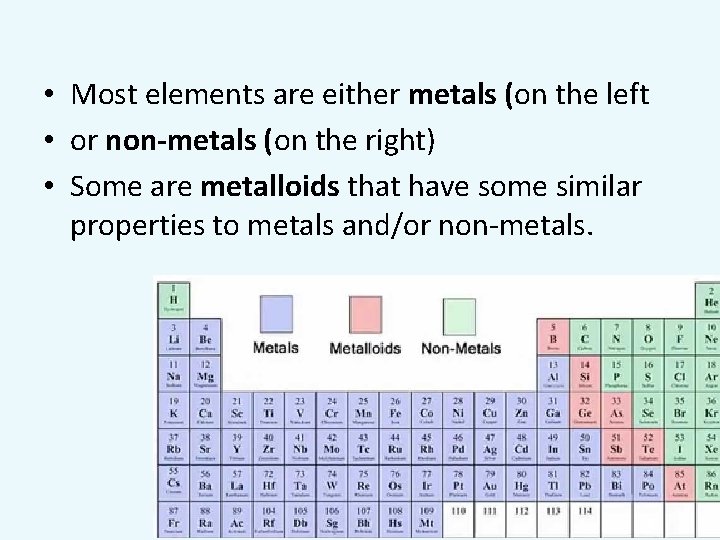
• Most elements are either metals (on the left • or non-metals (on the right) • Some are metalloids that have some similar properties to metals and/or non-metals.
Characteristics of Metals • • • Most elements are metals First 2 columns of PT Lustrous - shiny Malleable – can be pounded into a sheet Ductile – can be drawn into a wire Good conductors of heat and electricity. Solid at room temperature High density (are heavy for their size) High tensile strength (resist being stretched) High melting and boiling points.

Alkali Metals • • • First column (Group 1) of Periodic Table (but does not include Hydrogen. They all have one electron in their outer shell. shiny, soft and have low densities. some soft enough can be cut with a knife, Not found freely in nature because they all react vigorously with oxygen and water.

Alkali Metals • The reactivity of the alkali metals increases down the group • https: //www. youtube. com/watch? v=uixx. Jt. JPVXk

Alkali Metals • Flame tests are used to identify alkali metals Flame colour Ion present red lithium orange sodium lilac potassium

Interesting Facts • Because they are so reactive with air and water, they are generally stored in oil. • Cesium and rubidium are used to make atomic clocks. • They like to form salts by combining with halogens. • The name "alkali" is derived from the Arabic word for "ashes. " • Different alkali metals burn with different colored flames including sodium (orange yellow), lithium (red), potassium (lilac), rubidium (red), and cesium (blue or violet). • All alkali metals have odd atomic numbers. They are considered to be more similar to each other than any other group in the periodic table

• • Alkali metals are most reactive metals. That is the reason; their compounds are widely used in various fields and industries. Potassium and its compounds are used in the manufacturing of fertilizer, detergents, as explosives and also in photography industries. Rubidium and cesium are less useful compare to other alkali metals. Rubidium is used exclusively for research. Cesium is used in manufacturing of special glasses and radiation detection equipment. Potassium iodide with sodium chloride is used to cure the iodine deficiency. Sodium chloride with grit is used to prevent roads freezing in cold weather. Sodium chloride acts as water softener and electrolysis of it is used the manufacturing of Na. OH (Sodium hydroxide) which is one of the most important base and also chlorine gas. Sodium carbonate is the major ingredient of detergents and also used in manufacturing of glass and as water softener. Sodium bicarbonate is used as baking powder with tartaric acid.

Alkaline Earth Metals Group 2 of PT 2 electrons in valence shell Second most reactive family They are silvery, shiny, and relatively soft metals. • When mixed in solution they are likely to form “basic” or “alkaline” solutions. • •

Alkaline Earth Metals • only found in compounds and minerals, not in their elemental forms. • They react with halogens to form compounds called halides. • All except beryllium react strongly with water.

Interesting Facts • They burn with various colored flames as follows: beryllium (white), magnesium (bright white), calcium (red), strontium (crimson), barium (green), and radium (red). • The name "alkaline earths" comes from an old name for the oxides of the elements. They are called alkaline because they form solutions with a p. H greater than 7, making them bases or "alkaline. " • Radium is formed from the decay of uranium. It is very radioactive and is dangerous to handle. • Calcium and magnesium are important for animal and plant life. Calcium plays an important role in helping us to build strong bones and magnesium is used to help regulate the body's temperature. • Radium was discovered by scientists Marie and Pierre Curie.
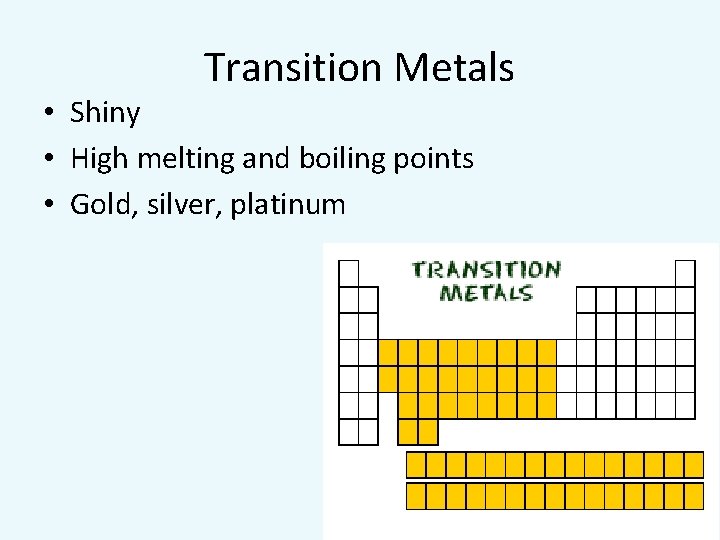
Transition Metals • Shiny • High melting and boiling points • Gold, silver, platinum

Transition Metals • They can form compounds with different colors. • They are metals and conduct electricity. • They have high melting and boiling points. • They have relatively high densities.
Interesting Facts • The transition metal group is called the "d-block" of the periodic table. • Iron, cobalt, and nickel are the only three elements that produce a magnetic field. • Chemists often use something called a "d electron count" instead of valence electrons to describe transition elements. • Because of their unique qualities, transition metals are often used in industry as catalysts for various reactions.
Non-metals • Elements that do not have the characteristics of metals are classified as non-metals. • They are found on the left side of the staircase of the Periodic Table. • Most non-metals typically share similar properties.

Non-metals: • • Hydrogen, Halogens, Noble Gases Generally gases or brittle, dull-looking solids Poor conductors of heat and electricity
Non-Metals • They are either gas (hydrogen, oxygen, nitrogen) or solid (carbon, sulfur) under standard conditions. • They are not good conductors of electricity or heat. • They are very brittle in their solid form. They are not malleable or ductile. • They generally have lower densities than metals. • They generally have lower melting and boiling points than metals. • The one exception to this is carbon.
Non-metals: Halogens • • Group 17 7 electrons in their outer shell very reactive typically found in minerals or salts

Uses of Halogen • Both chlorine and bromine are used as disinfectants for drinking water, swimming pools, fresh wounds, spas, dishes, and surfaces. They kill bacteria and other potentially harmful microorganisms through a process known as sterilization. • Chlorine and bromine are also used in bleaching.

Halogens • video
Noble Gases • Last column: Group Zero • Full valence shell • Inert (nonreactive) because they already have a stable octet. • Colorless, odorless gases. • Their melting and boiling points are close together giving them a very narrow liquid range. • Helium and Neon never form compounds, others form with great difficulty

Nobel Gas • Video • https: //www. youtube. com/watch? v=q. Na. BMv JXd. J 4

Interesting Facts • Krypton gets its name from the Greek word "kryptos" meaning "the hidden one. " • Many of the noble gases were either discovered or isolated by Scottish chemist Sir William Ramsay. • Helium has the lowest melting and boiling points of any substance. • Noble gases are often used to create a safe or inert atmosphere due to their stable nature. • Xenon gets its name from the Greek word "xenos" which means "stranger or foreigner. ".

Hydrogen: A special case • Usually placed on the left side of PT, but is a non-metal. • Often called a proton • Lightest element, • Colourless, tasteless, highly flammable gas • Most abundant (plentiful) element on Earth (Makes up over 90 % of the atoms in the universe).

Interesting Facts • Scientists estimate that Hydrogen makes up over 90 percent of all the atoms in the universe. • It is the only element that can exist without neutrons. • Hydrogen becomes a liquid at very low temperature and high pressure. • Under extremely high pressure it can become a liquid metal. It is thought that metallic hydrogen exists at the cores of gas giant planets like Jupiter. • Because it is so light, it was once used in lighter-thanair-balloons. However, it became too dangerous because of its highly flammable nature. Hydrogen gas can be produced in a lab by combining a dilute acid with a metal.

Helium • different from all of the other elements. • very stable with only two electrons in its valence shell. • But still grouped with the noble gases (8 valence e’s). • The noble gases and helium are all "happy, " because their valence shell is full.
Noble Gas • Neon
Noble Gas • Argon in light bulbs

Noble Gas • Xenon in head lamps of new cars

Semi-metals or Metalloids • Act like both metals and non-metals • Mostly metallic in their physical appearance: – Shiny solids at room temperature • mostly non-metallic in their chemical behavior. – brittle, not ductile – Poor conductors or semiconductors. • Examples include: boron, silicon, arsenic.
Check your understanding 1. Summarize characteristics of metals, nonmetals, and semi-metals. 2. What makes hydrogen an unusual element? 3. What characteristics define semi-metals?

Element Superhero
Modelling Atoms: Bohr Model • A Bohr Model is a simplified diagram of the number of electrons in each of the energy levels (shells) around an atom. • Each shell can only hold a certain number of electrons. • First shell can only hold a maximum of 2. • 2 nd shell = 8, 3 rd shell = 8, 4 th = 18. • The outermost shell is called the valence shell.
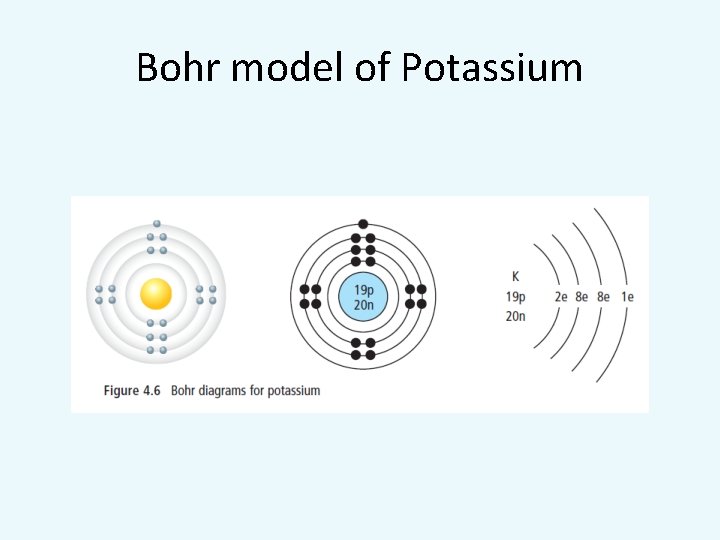
Bohr model of Potassium
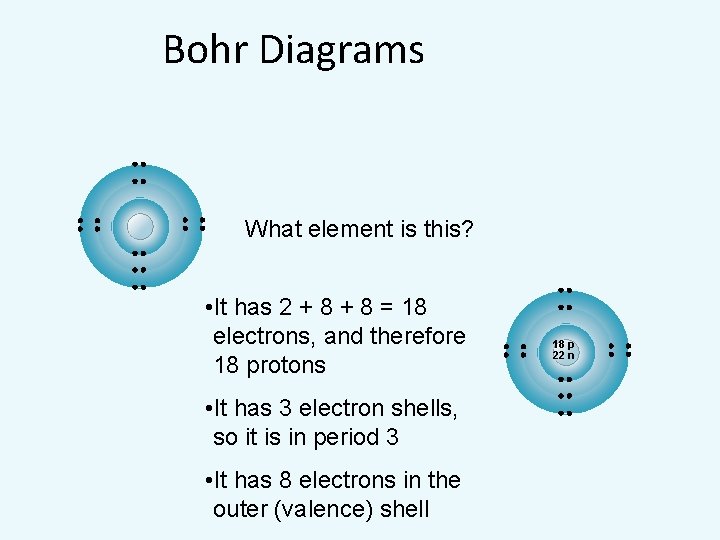
Bohr Diagrams What element is this? • It has 2 + 8 = 18 electrons, and therefore 18 protons • It has 3 electron shells, so it is in period 3 • It has 8 electrons in the outer (valence) shell 18 p 22 n
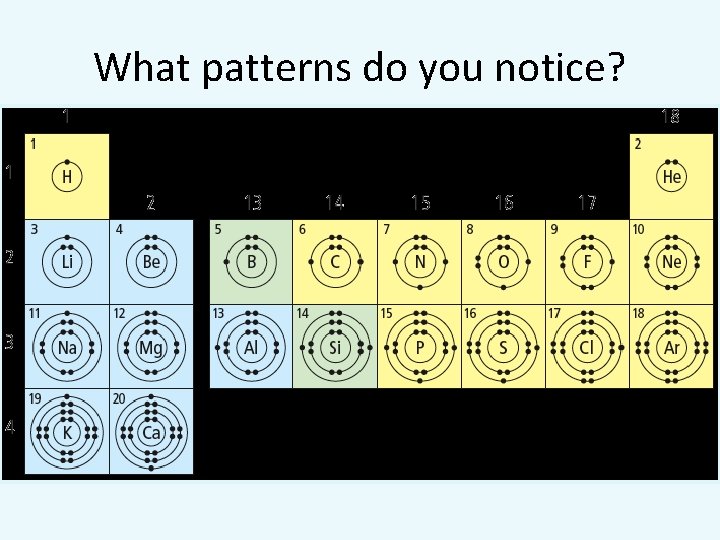
What patterns do you notice?
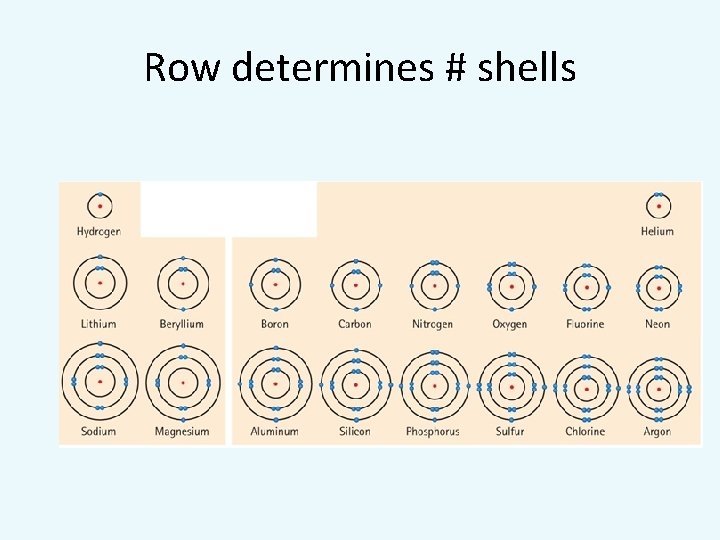
Row determines # shells
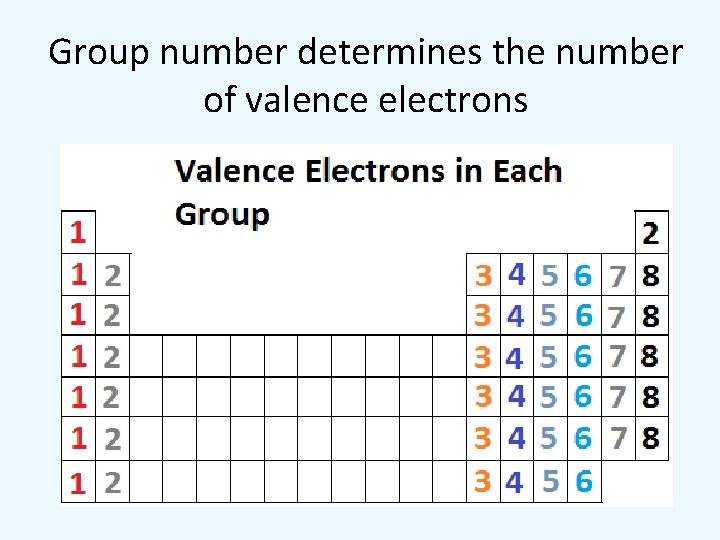
Group number determines the number of valence electrons
Patterns in the Periodic Table regarding electron arrangement 1. Elements of the same family have the same number of valence electrons 2. Elements that are in the same period have their valence electron in the same shell. 3. The period number of an element = the number of occupied energy shells of its atom.

How do atoms for ions? • An ion is an atom with an electric charge because it has gained or lost electrons from its valence shell. • An atom that loses one or more electrons positive ion (cation) • An atom that gains one or more electrons negative ion (anion)
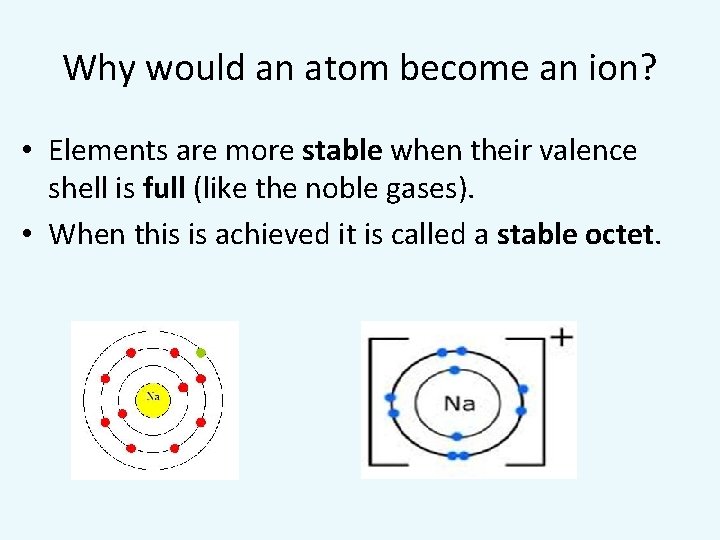
Why would an atom become an ion? • Elements are more stable when their valence shell is full (like the noble gases). • When this is achieved it is called a stable octet.
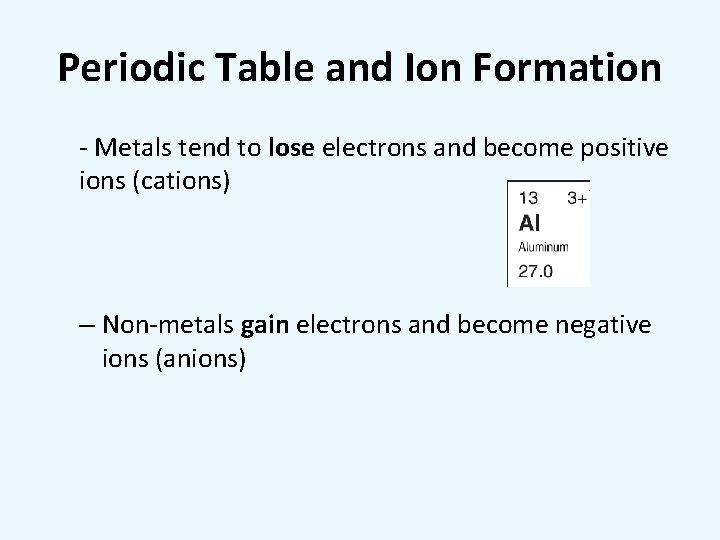
Periodic Table and Ion Formation - Metals tend to lose electrons and become positive ions (cations) – Non-metals gain electrons and become negative ions (anions)
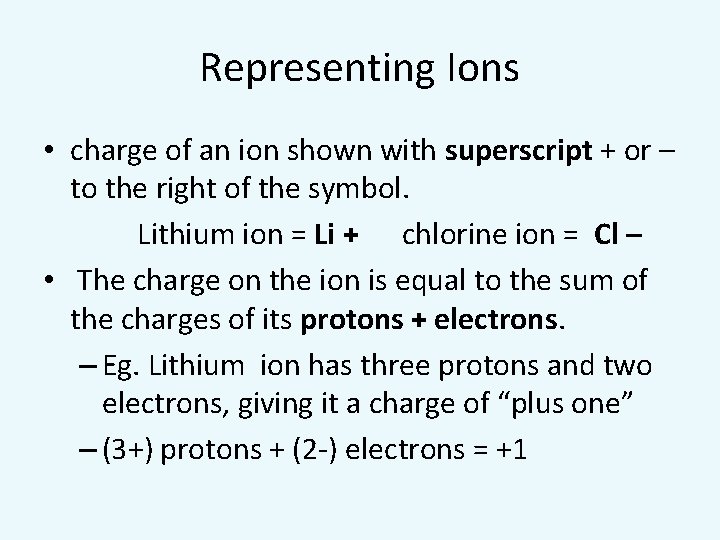
Representing Ions • charge of an ion shown with superscript + or – to the right of the symbol. Lithium ion = Li + chlorine ion = Cl – • The charge on the ion is equal to the sum of the charges of its protons + electrons. – Eg. Lithium ion has three protons and two electrons, giving it a charge of “plus one” – (3+) protons + (2 -) electrons = +1

Bohr Model of an Ion • Remember, an ion is an atom that has gained a charge (by either losing or gaining one or more electrons) • The Bohr model indicates the charge with a “+ or –” and brackets.
PRACTICE: Draw the Bohr model for an aluminium atom and ion. • aluminum-27 atom aluminium-27 ion
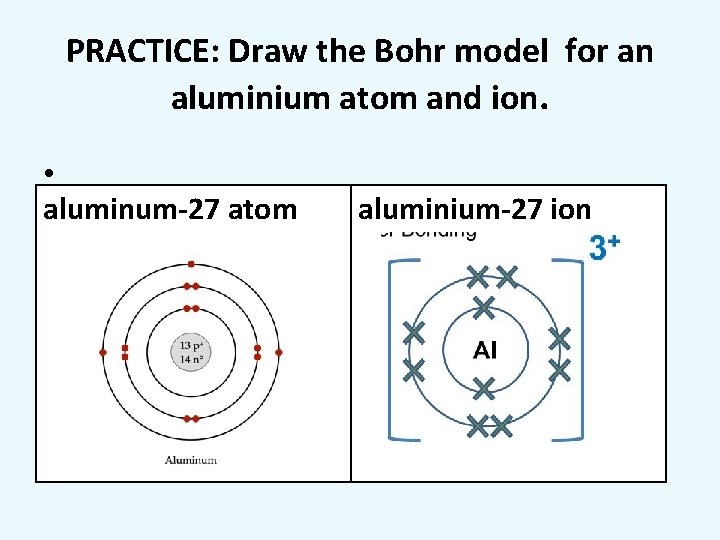
PRACTICE: Draw the Bohr model for an aluminium atom and ion. • aluminum-27 atom aluminium-27 ion
- Slides: 72