Periodic Table HISTORY GROUPS AND CHARACTERISTICS History of

Periodic Table: HISTORY, GROUPS, AND CHARACTERISTICS

(History of) The Periodic Table ▶ Mendeleev (Russian) – 1869, was the first person to arrange elements in order by weight ▶ Made “playing cards” ▶ Arranged by mass and saw repeating patterns in properties

(History of) The Periodic Table ▶ Moseley (English) – 1913, discovered that each element has an atomic number (protons) ▶ Organized based on this number ▶ Improved patterns of mendeleev ▶ Periodic Law – says that when elements are arranged in order by atomic number, their physical and chemical properties show a repeating pattern → every 8 elements.
So what does all this mean? Let’s look at your periodic Table

Basic Organization ▶ Groups/Families – vertical column ▶ – same # of valence electrons, which dictates their behavior (groups behave similarly) ▶ Period/Row – horizontal row ▶ – same outermost energy level, behavior changes predictably from left to right

Lets have some fun -Make a 1 by 6 chart. -Label the top row 1 st period, 2 nd row 2 nd period and so on until the sixth row and 6 th period - Put your name in one of the cells in 6 th period -Put down the name of a friend in each of your class periods (other than 6 th period). -a column is your school “family” or group and the row was your period. Easy right!

Check-In ▶ With your arms, show me where a Period is on the table. ▶ Again, with your arms – where is a Group?

But what can we figure out about our elements when we have a periodic table A lot actually let’s chat about it

Periodic characteristics Atomic Radius/ size increases as you go down ▶ 1. Atomic size – distance from the center of the atom (nucleus) to the outer edge of its electron cloud ▶ (measured by measuring the distance between the nuclei of two bonded atoms and dividing by two). Atomic Size
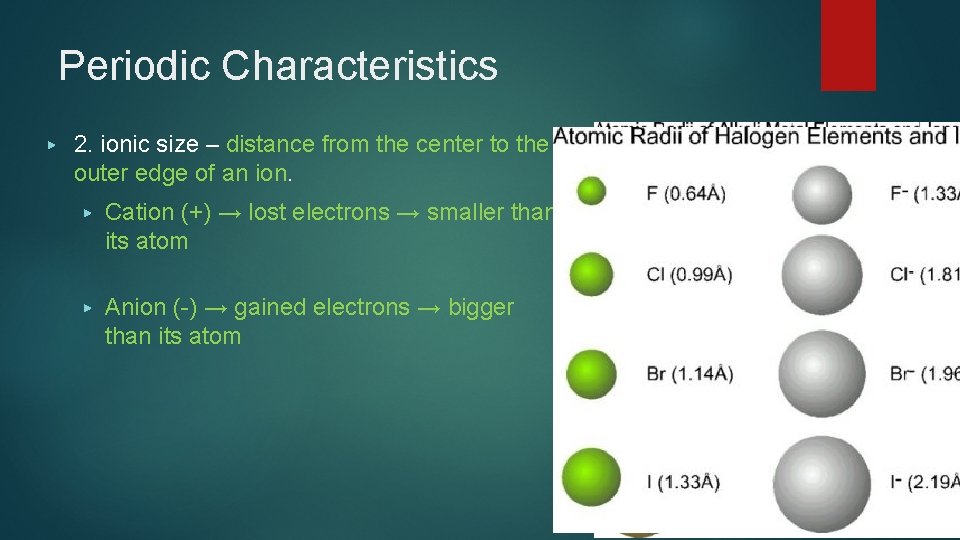
Periodic Characteristics ▶ 2. ionic size – distance from the center to the outer edge of an ion. ▶ Cation (+) → lost electrons → smaller than its atom ▶ Anion (-) → gained electrons → bigger than its atom

Check-In ▶ What will happen to Na when it makes an ion? (what will happen to its electrons? ) ▶ Will Na atom or Na ion be bigger? Why? ▶ What will happen to Cl when it makes an ion? (what will happen to its electrons? ) ▶ Will Cl atom or Cl ion be bigger? Why?

Let’s get this on our periodic table

Periodic Characteristics (cont. ) ▶ 3. Metallic properties ▶ ▶ ▶ Luster – shiny Conductivity – able to transfer heat or electrons Malleability – can be rolled or hammered into sheets Ductility – can be drawn (pulled) into a wire (like a specific version of malleable) Explained by: cations (bonding by) floating in a sea of mobile electrons ▶ Nonmetallic properties ▶ Luster – varies ▶ Poor conductor of heat and electricity ▶ Brittle ▶ Explained by: electrons are shared tightly in bonds

Let’s get this on your periodic table!

Check-In ▶ ▶ Tell your neighbor 3 characteristic properties of metals. They tell you 3 elements they would expect to have those properties. ▶ Tell your neighbor 3 properties of non-metals. ▶ They tell you 3 elements they would expect to have those properties.

Periodic Characteristics (cont. ) ▶ 4. Ionization Energy – energy needed to remove one of an atom’s electrons (1 st ionization energy is required to remove the first electron, 2 nd ionization energy is required to remove the second, etc. ) ▶ 5. Electronegativity – the ability of an atom to attract electrons in a chemical bond (think of it as how tightly the electrons are held). Noble gases don’t have electronegativity values because they don’t participate in bonds.

Check-In ▶ What is the very most Electronegative element on the whole table? ▶ Which is the least? ▶ Between N and O, which would you expect to have a higher Ionization Energy? ▶ Between Ca and Mg, which would you expect to have a higher IE?

Groups ▶ Elements in the same group have more similarities than elements in the same period because they have the same number of valence electrons.

Check-In ▶ As I point at groups on the periodic table, say out loud how many valence electrons that group has.

Alkali Metals (Group 1) ▶ Soft, can be cut with a knife ▶ Low density and melting points ▶ React violently with water and quickly with the oxygen in the air ▶ Never found uncombined in nature (always bonded to some other element) ▶ When they bond, always give away 1 electrons (making +1 ions)

Alkaline Earth Metals (Group 2) ▶ Soft (not as soft as Alkali Metals) ▶ Higher density and melting points than Group 1 ▶ Very reactive but not as much as Group 1 ▶ Not found uncombined in nature (also always bonded to another element) ▶ When they bond, always give away 2 electrons (making +2 ions)

Transition Metals ▶ Metals with higher densities and boiling points ▶ Variable properties across the group ▶ In the d-block ▶ Very flexible with their electrons (leading to their variable properties) ▶ Still metals – tend to give away some number of electrons making (+) ions

Metalloids (say with a robot accent) ▶ Can behave more like metals or nonmetals depending on the environment they are in ▶ Touch the stairstep line on the periodic table: B, Si, Ge, As, Sb, Te

Diatomic Elements ▶ HONCl. Br. IF (or Br. INCl. HOF) elements – found combined with self (Br 2, I 2, Cl 2, etc) ▶ Never appear as just one atom, if not combined with something else, they bond with another atom of the same element.

Halogens (Group 17) ▶ Form salt compounds with metals ▶ Exist as diatomic molecules ▶ Highly reactive ▶ Not free elements in nature ▶ I 2 is a solid at room temperature, Br 2 is a liquid, and Cl 2 and F 2 are gases ▶ Predictably gain one electron, making (-1) ions

Noble Gases (Group 18) ▶ Least reactive of the elements (vocab word: INERT means non-reactive) ▶ All have full valence shell (which is why they’re least reactive) ▶ All gases at room temperature
- Slides: 26