Periodic Table History Dobereiner He put elements with

Periodic Table History

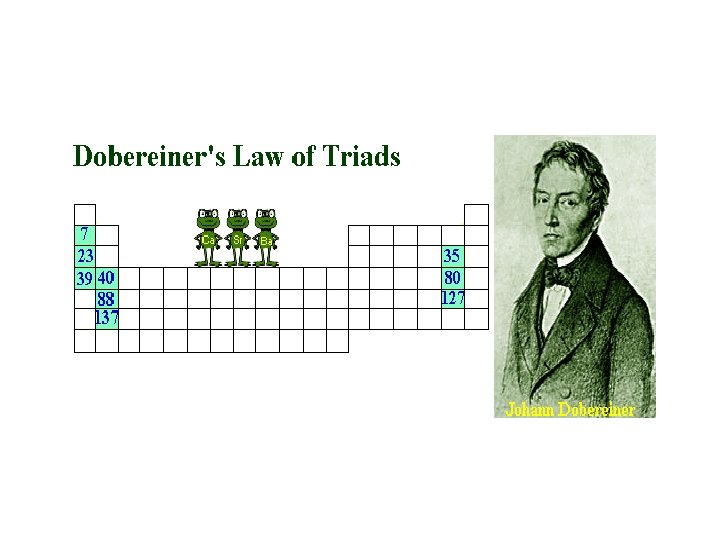
Dobereiner • He put elements with similar chemical properties together • They went into in groups of 3 • He noticed these trends in groups of elements such as: Can you name these elements ? Atomic Weight of “middle” element is average of other two – approx.

Dobereiner’s Law of Triads • A triad is a group of three elements • with similar chemical properties in which • the atomic mass of the middle element is approximately equal to the average of the other two. • Only worked for very few elements of the 50 or so that were known at the time • BUT • He was first to make a link between atomic weight and properties

John Newland • Arranged the 60 known elements in order of increasing atomic weight • His pattern was… • Every 8 th element was a repeat (i. e. had 7 groups) • His Law was called…

Law of Octaves • An octave is a group of elements arranged in order of increasing atomic weight, in which the first and the eighth element of each group have similar properties. • The properties repeat every 8 as noble gases hadn’t been discovered yet! • Only worked for 17 out of 60 elements • There were several problems such as iron being grouped with oxygen and sulphur. • Laughed at but was basically correct

Mendeleev • Arranged the known elements in order of increasing atomic weight • His Law was called…

Mendeleev’s Periodic Law: • When elements are arranged in order of increasing atomic weight (relative atomic mass), the properties of the elements vary periodically.
Mendeleev: • Put elements with the same properties in the same vertical group. • Reversed the order of some elements (Te/I) so that their properties matched their group. • Left gaps to make the elements fit into the proper column (group). • Predicted that elements (eg. Germanium and Gallium) would be discovered to fill these gaps. Predicted their properties correctly.
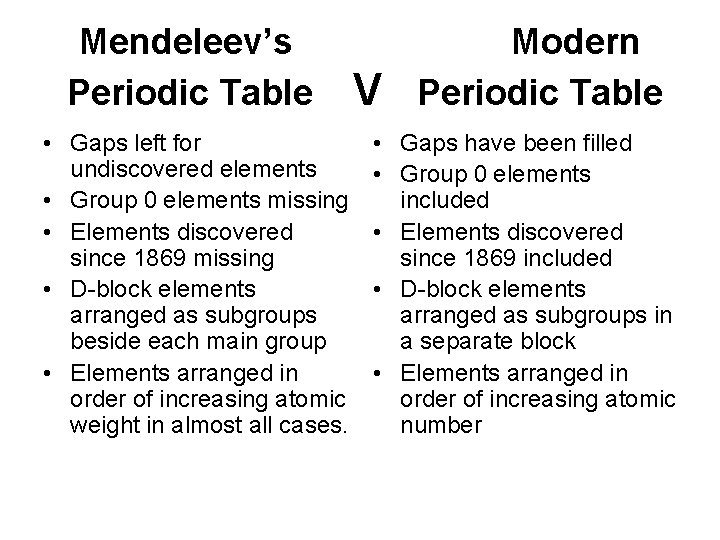
Mendeleev’s Periodic Table • Gaps left for undiscovered elements • Group 0 elements missing • Elements discovered since 1869 missing • D-block elements arranged as subgroups beside each main group • Elements arranged in order of increasing atomic weight in almost all cases. V Modern Periodic Table • Gaps have been filled • Group 0 elements included • Elements discovered since 1869 included • D-block elements arranged as subgroups in a separate block • Elements arranged in order of increasing atomic number

Mendeleev’s table

Moseley – Atomic Number • The atomic number of an atom is the number of protons in the nucleus of that atom. • Moseley used x-rays to find out how much positive charge each nucleus had • In other words the difference between the elements is the number of protons in the nucleus. • Once the atomic number was known it was seen that Mendeleev’s table was in order of increasing atomic number – not weight.

Modern Periodic Table • In order of increasing atomic number. • There were 63 elements in Mendeleev’s table – now 109 • No gaps • The transition elements are listed separately. • Today’s table 1940 – Glenn Seaborg

Do I know it? ? ? • • • Who proposed Law of Triads? Who proposed Law of Octaves? Who proposed Periodic Law? Can I explain each ? Can I name 3 triads? What was Moseley’s contribution to the Periodic Table development? • Four differences between Mendeleev’s Table and modern table?
- Slides: 14