Periodic Table History and Development History of the

Periodic Table History and Development

History of the Periodic Table Mendeleev (1860’s) – Developed the first periodic table – It was arranged by atomic mass – He was able to predict properties of elements Moseley - developed the modern periodic table, arranged by atomic number (number of protons) Memory Trick: M & M created the periodic table…
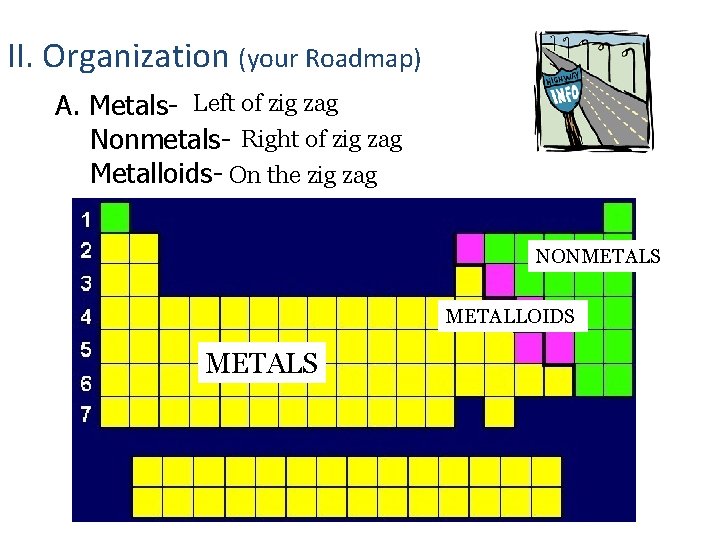
II. Organization (your Roadmap) A. Metals- Left of zig zag Nonmetals- Right of zig zag Metalloids- On the zig zag NONMETALS METALLOIDS METALS
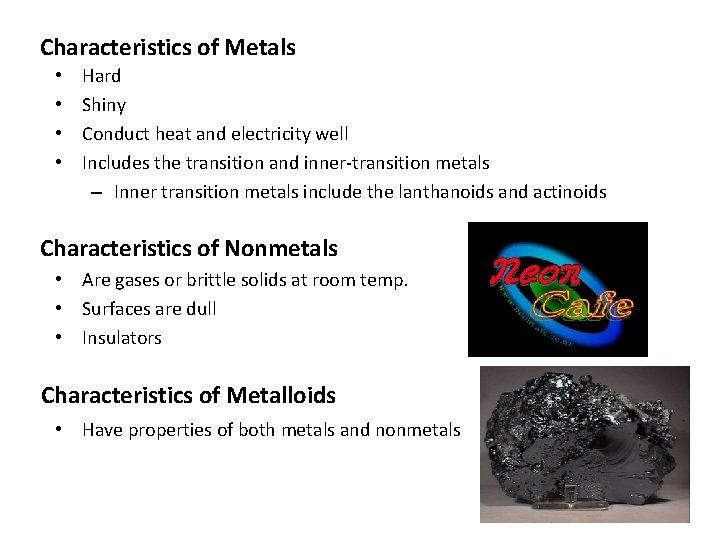
Characteristics of Metals • • Hard Shiny Conduct heat and electricity well Includes the transition and inner-transition metals – Inner transition metals include the lanthanoids and actinoids Characteristics of Nonmetals • Are gases or brittle solids at room temp. • Surfaces are dull • Insulators Characteristics of Metalloids • Have properties of both metals and nonmetals
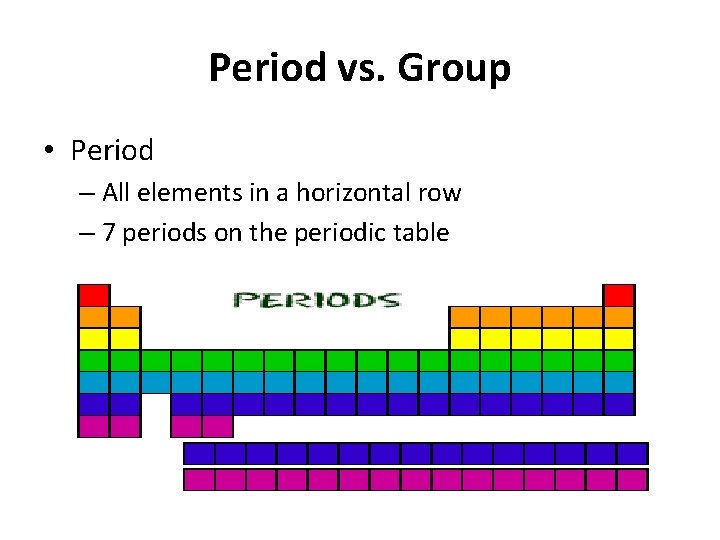
Period vs. Group • Period – All elements in a horizontal row – 7 periods on the periodic table
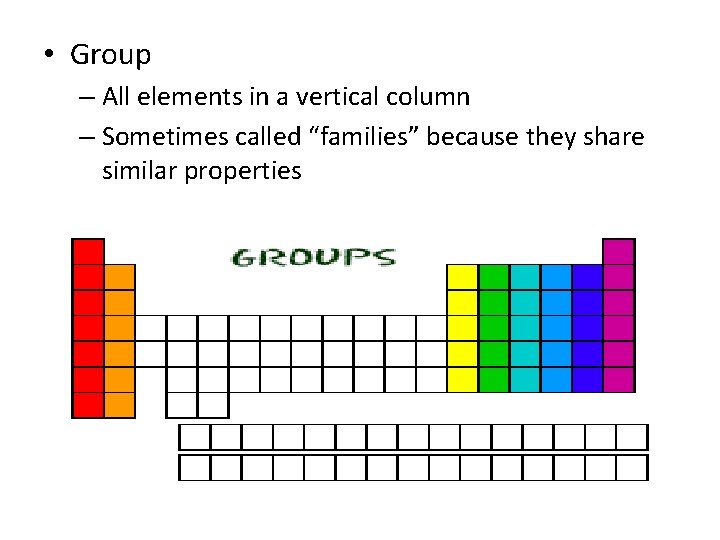
• Group – All elements in a vertical column – Sometimes called “families” because they share similar properties

Families you should know… Group 1 (IA) – Alkali Metals Group 2 (IIA) – Alkaline Earth Metals Group 17 (VIIA) - Halogens Group 18 (VIIIA) – Noble Gases
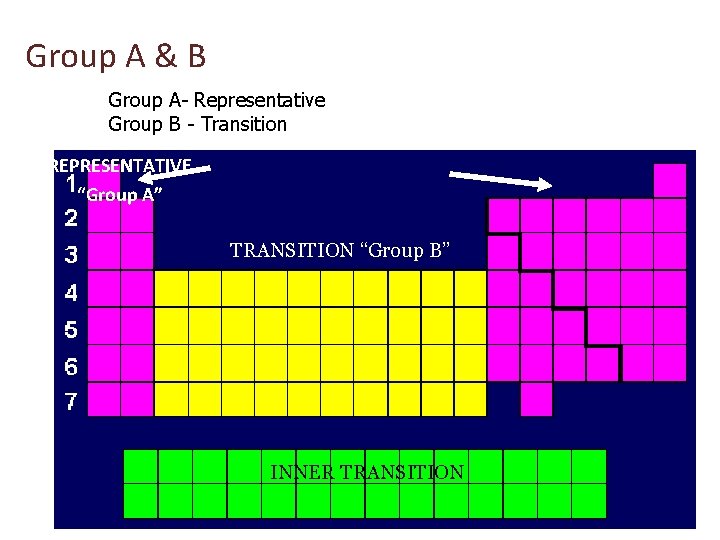
Group A & B Group A- Representative Group B - Transition REPRESENTATIVE “Group A” TRANSITION “Group B” INNER TRANSITION
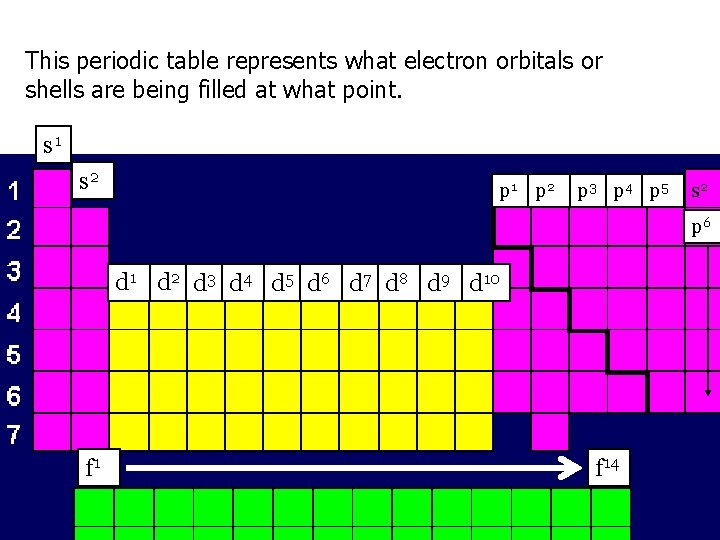
This periodic table represents what electron orbitals or shells are being filled at what point. s 1 s 2 p 1 p 2 p 3 p 4 p 5 s 2 p 6 d 1 d 2 d 3 d 4 d 5 d 6 d 7 d 8 d 9 d 10 f 14
- Slides: 9