Periodic Table Families Adapted from Liz La Rosa

Periodic Table Families Adapted from Liz La. Rosa Some images are from www. chem 4 kids. com www. middleschoolscience. com 2008
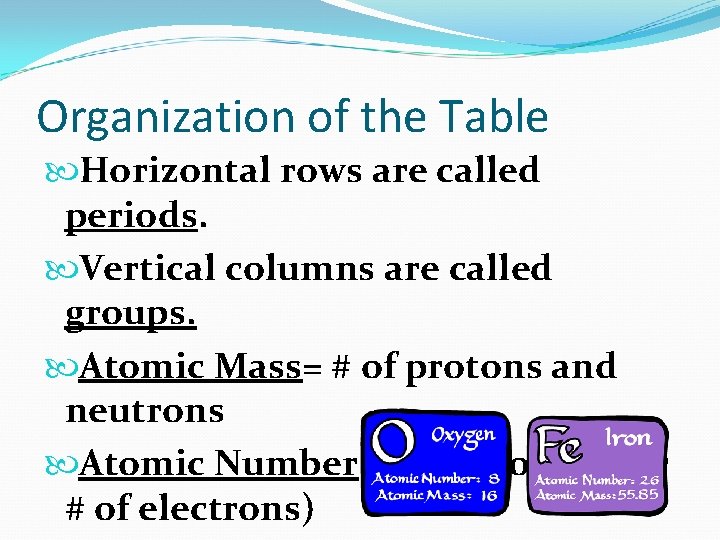
Organization of the Table Horizontal rows are called periods. Vertical columns are called groups. Atomic Mass= # of protons and neutrons Atomic Number = # of protons (or # of electrons)

Characteristics of Metals Luster (shiny) Ductility (ability to be pulled into thin wires) Malleability (ability to be hammered) Conductor Almost all are solid at room temperature
Characteristics of Nonmetals Many are gases at room temperature. Solids are brittle No luster (dull) Good insulators

Characteristics of Metalloids Properties of both metals and nonmetals Semiconductor – conducts electricity at high temperatures, but not at low temperatures.

Discussion and Draw Compare and contrast metals and nonmetals.
Families on the Periodic Table Elements on the periodic table can be grouped into families bases on their chemical properties. Each family has a specific name to differentiate it from the other families in the periodic table. Elements in each family react differently with other elements.

Hydrogen belongs to a family of its own. Hydrogen is a diatomic, reactive gas. Hydrogen was involved in the explosion of the Hindenberg. Hydrogen is promising as an alternative fuel source for automobiles
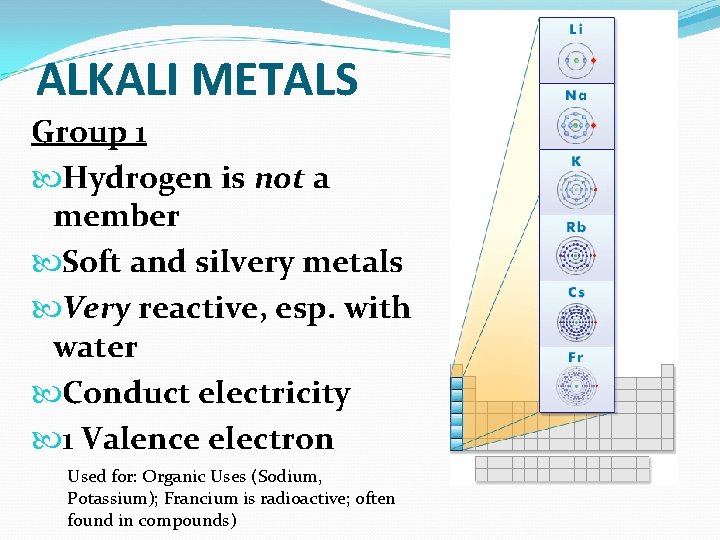
ALKALI METALS Group 1 Hydrogen is not a member Soft and silvery metals Very reactive, esp. with water Conduct electricity 1 Valence electron Used for: Organic Uses (Sodium, Potassium); Francium is radioactive; often found in compounds)
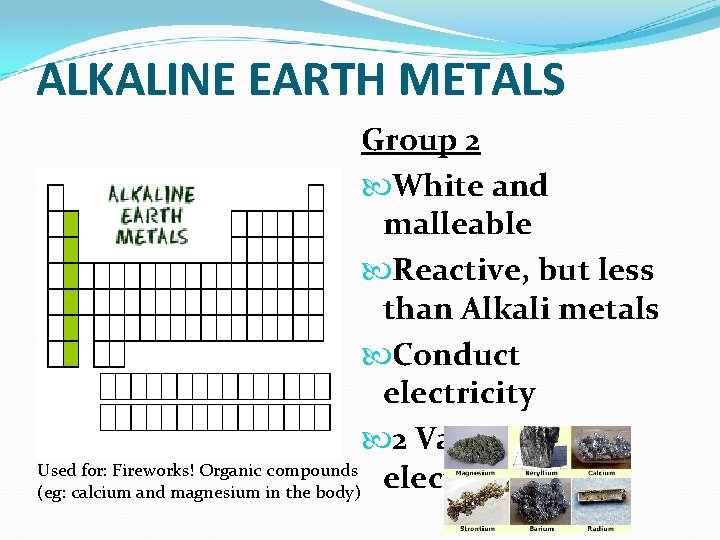
ALKALINE EARTH METALS Group 2 White and malleable Reactive, but less than Alkali metals Conduct electricity 2 Valence Used for: Fireworks! Organic compounds electrons (eg: calcium and magnesium in the body)
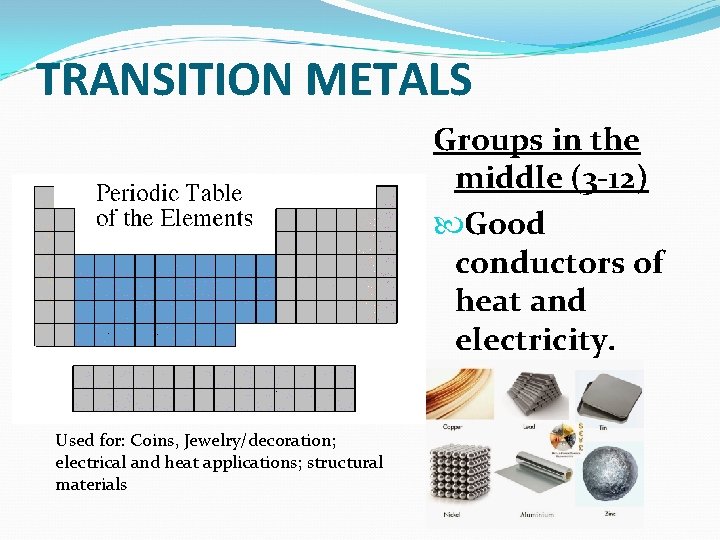
TRANSITION METALS Groups in the middle (3 -12) Good conductors of heat and electricity. Used for: Coins, Jewelry/decoration; electrical and heat applications; structural materials
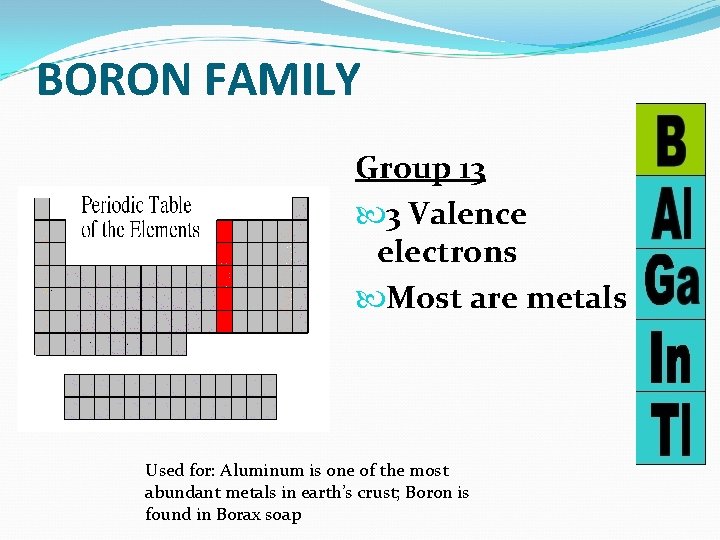
BORON FAMILY Group 13 3 Valence electrons Most are metals Used for: Aluminum is one of the most abundant metals in earth’s crust; Boron is found in Borax soap
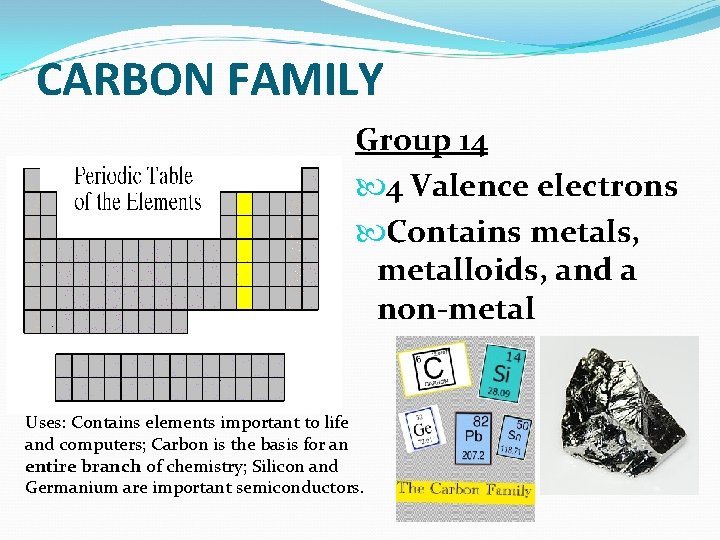
CARBON FAMILY Group 14 4 Valence electrons Contains metals, metalloids, and a non-metal Uses: Contains elements important to life and computers; Carbon is the basis for an entire branch of chemistry; Silicon and Germanium are important semiconductors.
NITROGEN FAMILY Group 15 5 Valence Electrons Contains metals, metalloids, and non-metals Uses: Nitrogen makes up over ¾ of the atmosphere; Nitrogen and phosphorus are both important in living things.
OXYGEN FAMILY (aka: Chalcogens) Group 16 6 Valence electrons Contains metals, metalloids, and non-metals Reactive Uses: Oxygen is necessary for respiration. Many things that stink, contain sulfur (rotten eggs, garlic, skunks, etc. )
Halogens Group 17 7 Valence electrons All are non-metals Very reactive react with metals to form salt often bonds with Group 1 Uses: Always found combined with other element in nature; Used as disinfectants and to strengthen teeth.
Noble Gases Uses: Used in lighted “neon” signs Used in blimps to fix the Hindenberg problem. Group 18 Gases Non-metals 8 Valence electrons = Full (except He = 2) Not reactive with other elements
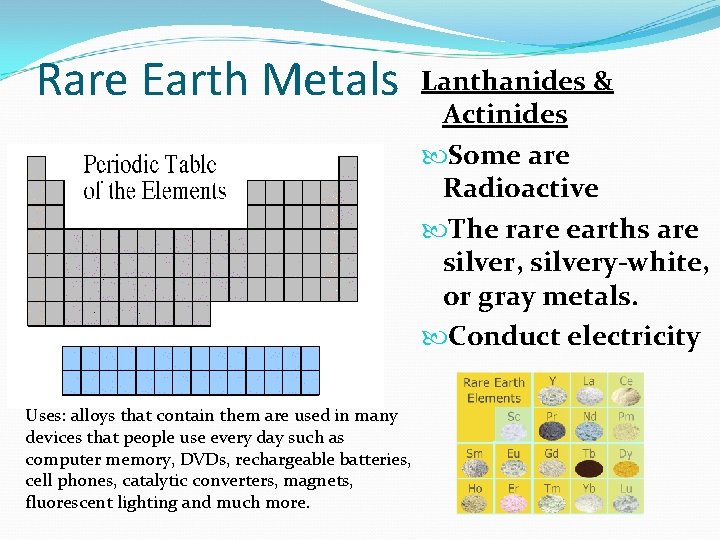
Rare Earth Metals Uses: alloys that contain them are used in many devices that people use every day such as computer memory, DVDs, rechargeable batteries, cell phones, catalytic converters, magnets, fluorescent lighting and much more. Lanthanides & Actinides Some are Radioactive The rare earths are silver, silvery-white, or gray metals. Conduct electricity

Periodic Table Families Review Period: Shells: Group: Valence Electrons: Metals: Non Metals: Metalloids:

https: //create. kahoot. it/details/chemistry-periodictable-families/82238769 -ba 1 e-467 c-836 bc 73430 b 66783

Discussion What patterns can you identify on the periodic table? Provide evidence.
Learning Scale – I can … 4 – Differentiate between the various groups on the periodic table. 3 - Describe the various groups on the periodic table. 2 - Use the periodic table to determine the number of protons, neutrons, or electrons. 1 - Identify patterns on the periodic table.
- Slides: 22