Periodic Table Essential Question What are the trends

Periodic Table

Essential Question? ? ? What are the trends observed in the periodic table and how is it arranged?

Mendeleev 1869: Mendeleev created the first periodic table; arranged by atomic mass ; arranged by similar properties

Moseley 1913: Moseley used X-rays to find # of protons; arranged by atomic # (# protons) ; arranged by similar properties
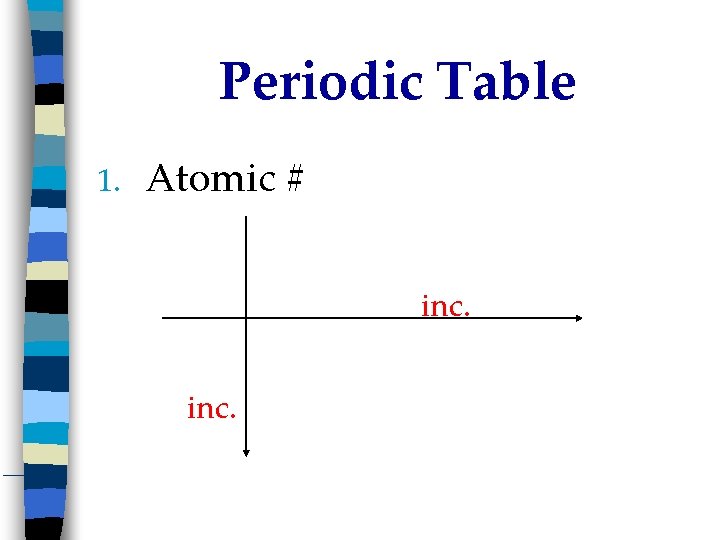
Periodic Table 1. Atomic # inc.
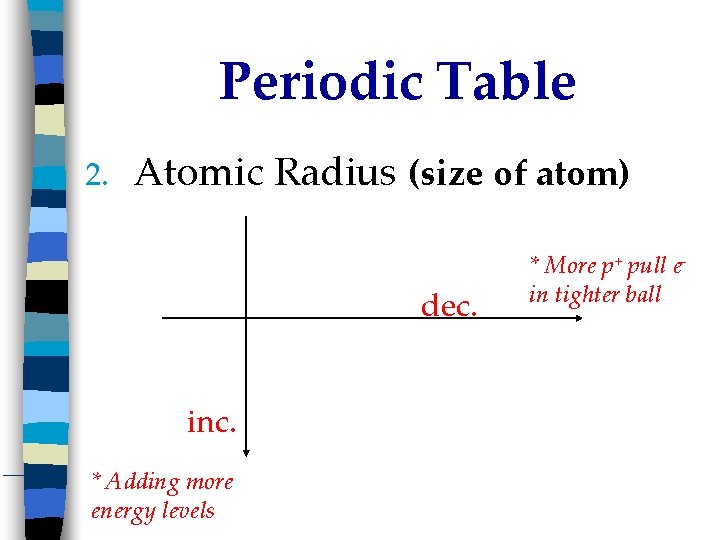
Periodic Table 2. Atomic Radius (size of atom) dec. inc. * Adding more energy levels * More p+ pull ein tighter ball
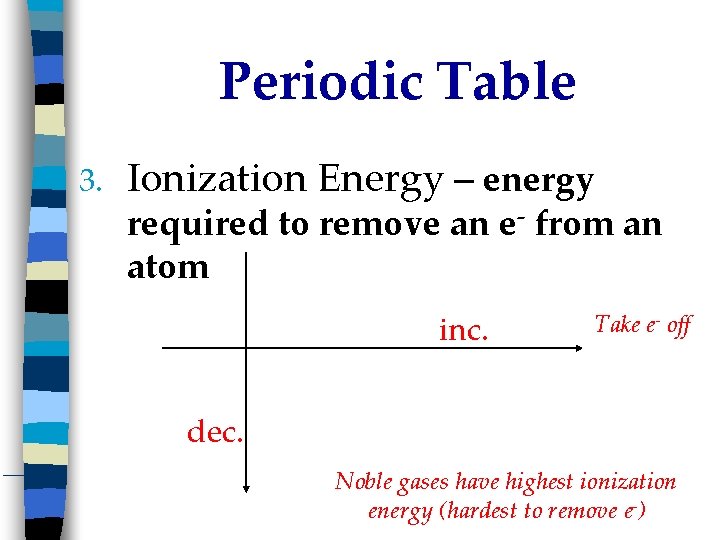
Periodic Table 3. Ionization Energy – energy required to remove an e- from an atom inc. Take e- off dec. Noble gases have highest ionization energy (hardest to remove e-)
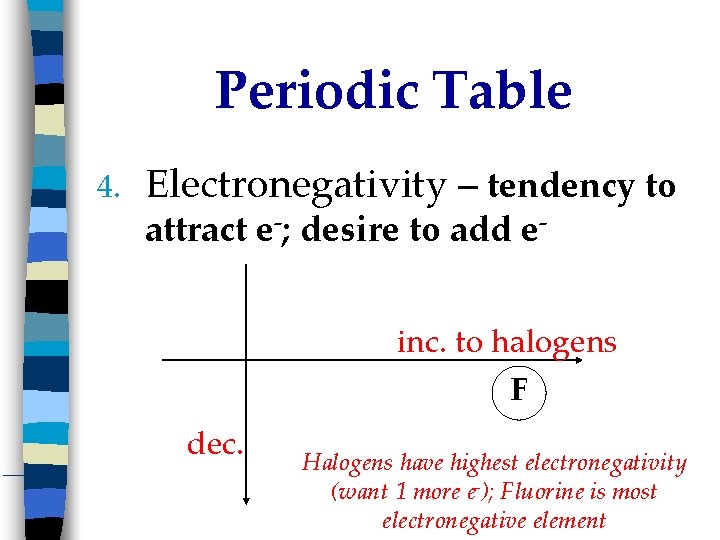
Periodic Table 4. Electronegativity – tendency to attract e-; desire to add e- inc. to halogens F dec. Halogens have highest electronegativity (want 1 more e-); Fluorine is most electronegative element
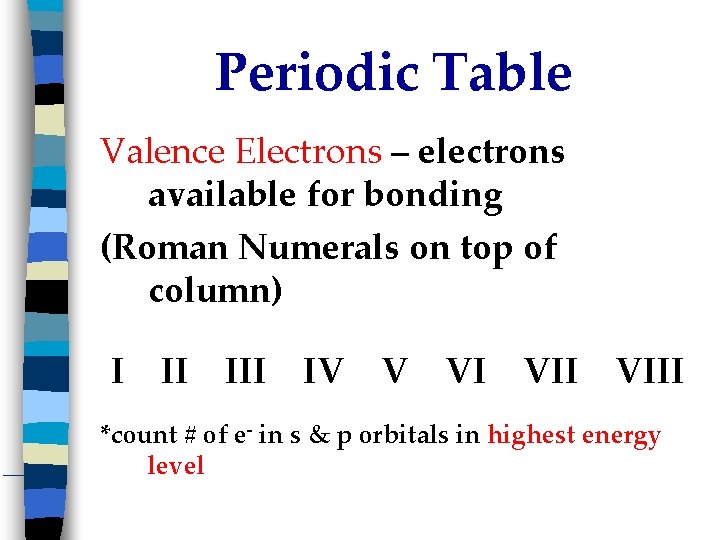
Periodic Table Valence Electrons – electrons available for bonding (Roman Numerals on top of column) I II IV V VI VIII *count # of e- in s & p orbitals in highest energy level

Periodic Table Metals Left of zig zag on periodic table Luster (shiny) Conducts heat and electricity Malleable – bendable; can hammer into sheets Ductile – pull into wires + charges (lose electrons)

Periodic Table Non-metals Right of zig zag on periodic table Usually gases Can also be brittle, chalky solids that come in a variety of colors Does not conduct heat or electricity - charges (gain electrons)
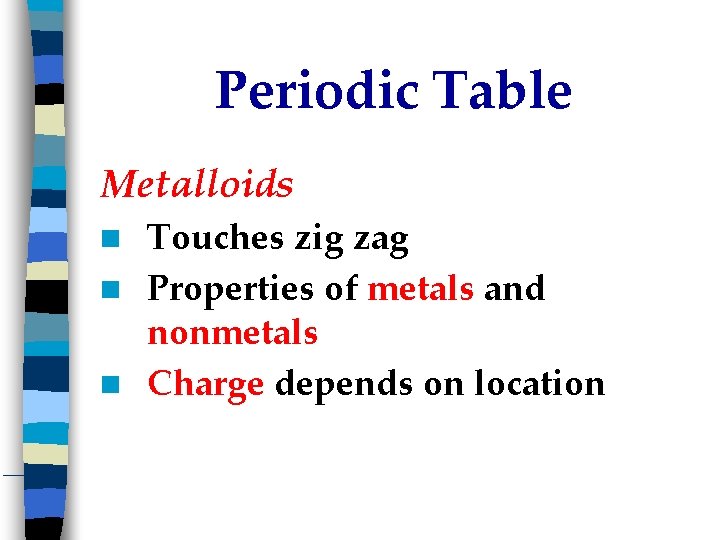
Periodic Table Metalloids Touches zig zag Properties of metals and nonmetals Charge depends on location
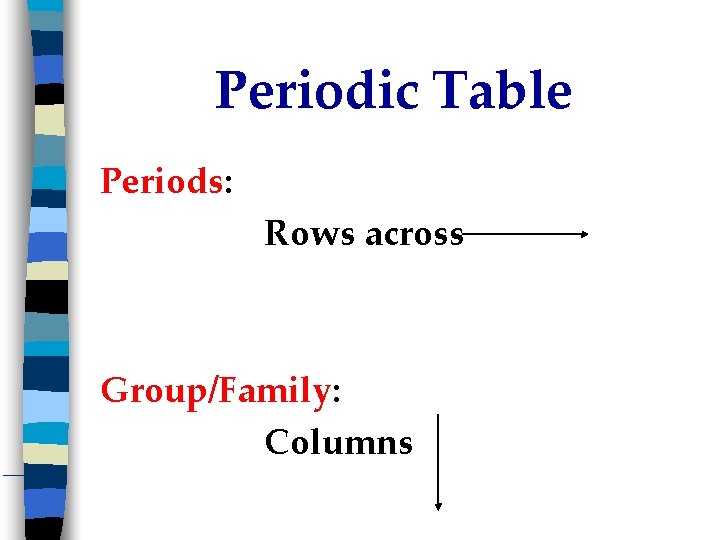
Periodic Table Periods: Rows across Group/Family: Columns
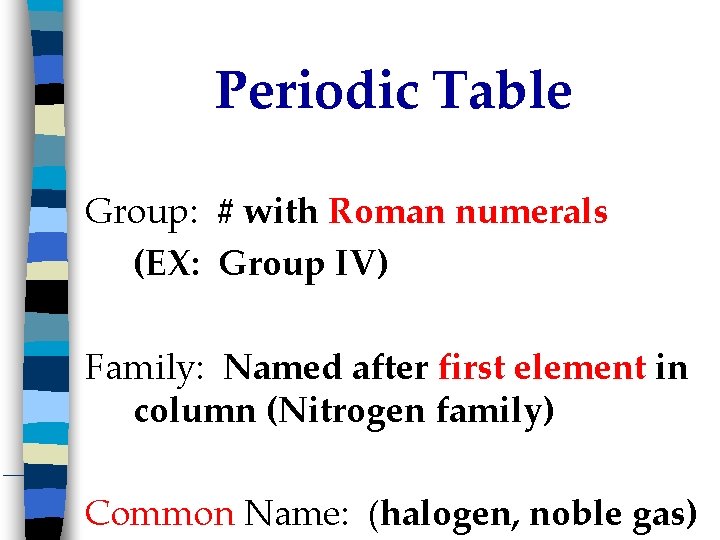
Periodic Table Group: # with Roman numerals (EX: Group IV) Family: Named after first element in column (Nitrogen family) Common Name: (halogen, noble gas)

Wrap-Up Write 2 questions over the material covered today. Write a summary over the notes. Remember to think about the essential question (What are the trends observed in the periodic table and how is it arranged? )
- Slides: 15