Periodic Table Elements Greek philosophers decided that the
Periodic Table

Elements • Greek philosophers decided that the universe was made up of basic elements • They said these elements were earth, air, fire and water • Formed the basis of chemical theory for almost 2, 000 years……

• First accurate description of an element given by Robert Boyle • An element is a substance that cannot be split into simpler substances by chemical means • Boyle predicted that elements combine in chemical reactions to form compounds
Humphry Davy • Studied the effect of passing electricity through different substances • Isolated the elements potassium, sodium and calcium through electrolysis
Dobereiner’s Triads • Noticed that bromine seemed halfway in its properties between iodine and chlorine • Br is liquid, I is solid, Cl is gas • Observed that the atomic weight of bromine is about halfway between chlorine and iodine

• Other triads: calcium, strontium, barium. Sulphur, selenium, tellurium • A triad is a group of elements with similar chemical properties in which the atomic weight of the middle element is approximately halfway between the other two
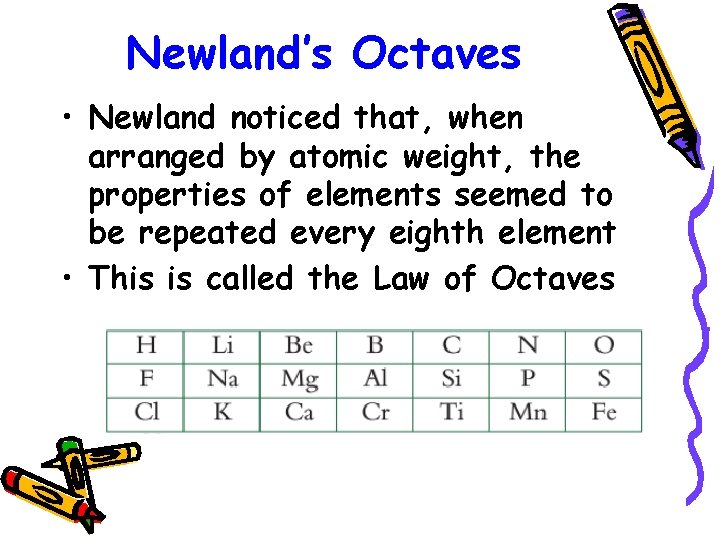
Newland’s Octaves • Newland noticed that, when arranged by atomic weight, the properties of elements seemed to be repeated every eighth element • This is called the Law of Octaves

• Newlands’ Law of Octaves breaks down after Ca. • Newlands did not realise that there were some elements yet to be discovered
Mendeleev’s Periodic Law • When elements are arranged according to atomic weight, their properties repeat periodically
• Left gaps in the table to make the elements “fit” • Reversed the order of some elements to make them “fit”
Henry Moseley and the modern periodic table • Using x-ray analysis, Moseley discovered the atomic number • Atomic number is the number of protons in the nucleus • Rearranged Mendeleev’s table according to atomic number
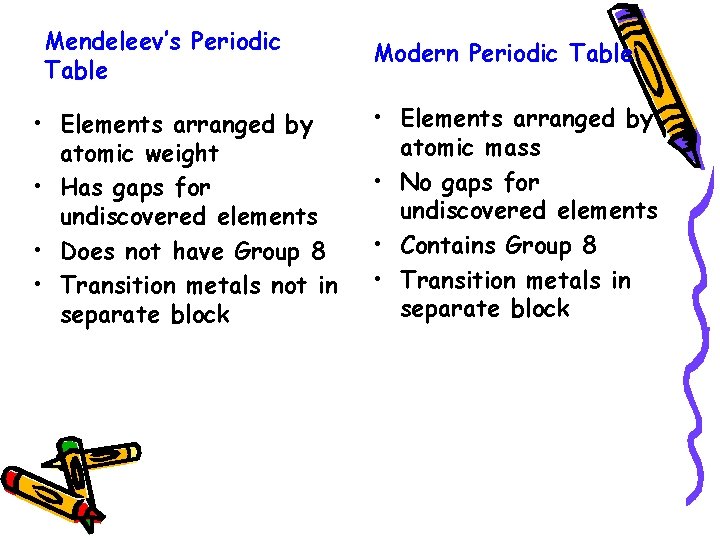
Mendeleev’s Periodic Table • Elements arranged by atomic weight • Has gaps for undiscovered elements • Does not have Group 8 • Transition metals not in separate block Modern Periodic Table • Elements arranged by atomic mass • No gaps for undiscovered elements • Contains Group 8 • Transition metals in separate block

Atomic Number • Henry Moseley discovered that each element has its own Atomic Number (Z) • Atomic Number tells us: – Number of protons – Number of electrons – Place in the periodic table
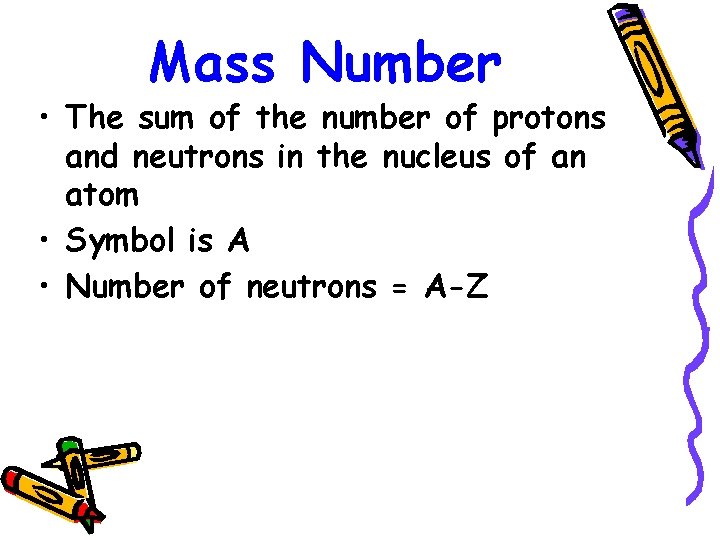
Mass Number • The sum of the number of protons and neutrons in the nucleus of an atom • Symbol is A • Number of neutrons = A-Z
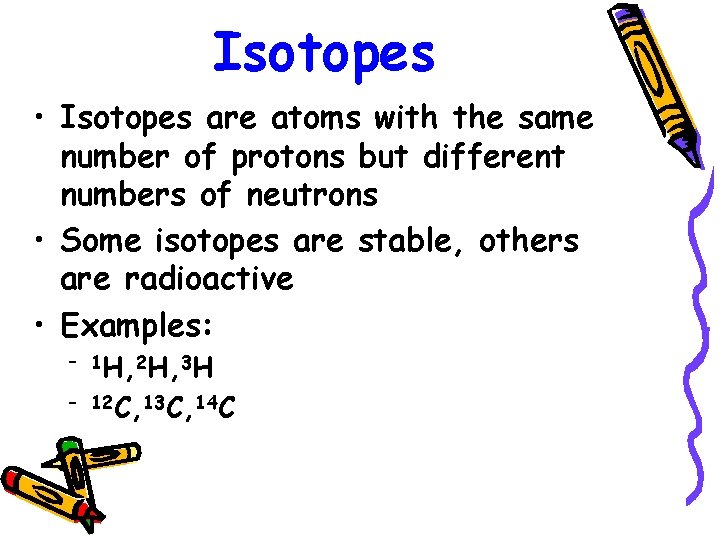
Isotopes • Isotopes are atoms with the same number of protons but different numbers of neutrons • Some isotopes are stable, others are radioactive • Examples: – 1 H, 2 H, 3 H – 12 C, 13 C, 14 C
Relative Atomic Mass • Relative Atomic Mass (Ar) is the average mass of an atom relative to one-twelfth the mass of an atom of carbon-12 • Relative atomic mass takes the abundance of an isotope into account
- Slides: 18