Periodic Table Charges and Ionic Compounds Learning Targets

Periodic Table, Charges, and Ionic Compounds

Learning Targets • LT 1 I can give the symbol for any element, number 1 -20 on the periodic table, given its name • LT 2 I can give the name for any element, number 1 -20, given its symbol • LT 3 I can name the groups/families on the periodic table • LT 4 I can give the charge made when a given element is ionized, based on its group • LT 5 I can identify 9 common polyatomic ions by their name, formula, and their charge • LT 6 I can write a balanced Ionic formula given the name of an ionic compound • LT 7 I can write the name of an ionic compound, given its ionic formula

Alkalai Metals • Always form a +1 charge • Are very reactive with water • Reactivity with water increases as you go down the group

Alkaline Earth Metals • Always form a +2 charge • Are also reactive with water, but less so than the alkalai metals
Transition Metals • Form several charges, you’ll have to look them up or figure it out based on the ionic formula
Nobel Gases • Do not form ions • Do not react with other elements except under EXTREME circumstances
Halogens • Always form a -1 charge • When they’re on their own, they’re diatomic

(aside) Diatomic Molecules • Some elements, when alone, will pair up with themselves • Remember HONCl. Br. IF • H 2, O 2, N 2, Cl 2, Br 2, I 2, F 2
Column 6 (misc) • Always form a -2 charge • Properties change a lot as you go down the group
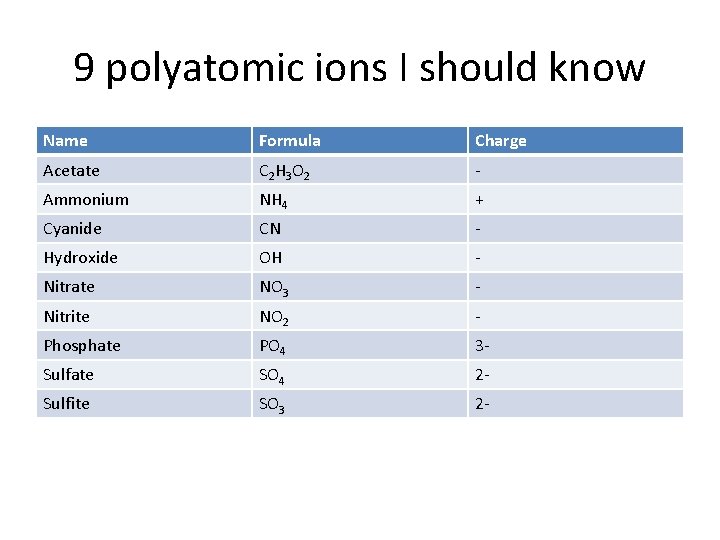
9 polyatomic ions I should know Name Formula Charge Acetate C 2 H 3 O 2 - Ammonium NH 4 + Cyanide CN - Hydroxide OH - Nitrate NO 3 - Nitrite NO 2 - Phosphate PO 4 3 - Sulfate SO 4 2 - Sulfite SO 3 2 -

Balancing Ionic Formulas • The formulas for ionic compounds must be balanced based on the charges of the ions • Cations will give a positive charge, anions will give a negative charge, the overall charge must be zero. • This is done by changing the subscripts of the cation and anion • Polyatomic ions should be put in parenthesis if they need a subscript

Balancing Ionic Formulas, example Ex: aluminum sulfate Aluminum is Al, sulfate is SO 4, so Al. SO 4 is where we start Aluminum makes a 3+ charge, sulfate makes a 2 - charge. To balance the charges, we need positive and negative to equal each other. So, we need a total positive charge of 6 and a total negative charge of 6, since 6 is the smallest number with 2 and 3 as factors • To make Aluminum’s +3 into +6, we need to multiply it by 2, so we add the subscript: Al 2 SO 4. • To make sulfate’s -2 into -6, we need to multiply it by 3, so we add the subscript (note, because sulfate is polyatomic, we put it in parenthesis): Al 2(SO 4)3 • •

Naming Ionic Compounds • Start with the cation (positive charge) • End with the anion (negative charge) • Change the ending of an anion to –ide – Ex: Na. Cl is not sodium chlorine, it’s sodium chloride • Polyatomic Ions don’t have their ending changed – Ag. NO 3 is silver nitrate
- Slides: 15